
Open hearth furnace
Encyclopedia
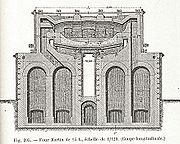
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
where excess carbon and other impurities are burnt out of the pig iron
Pig iron
Pig iron is the intermediate product of smelting iron ore with a high-carbon fuel such as coke, usually with limestone as a flux. Charcoal and anthracite have also been used as fuel...
to produce steel
Steelmaking
Steelmaking is the second step in producing steel from iron ore. In this stage, impurities such as sulfur, phosphorus, and excess carbon are removed from the raw iron, and alloying elements such as manganese, nickel, chromium and vanadium are added to produce the exact steel required.-Older...
. Since steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
is difficult to manufacture due to its high melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
, normal fuels and furnaces were insufficient and the open hearth furnace was developed to overcome this difficulty. Most open hearth furnaces were closed by the early 1990s, not least because of their slow operation, being replaced by the basic oxygen furnace or electric arc furnace
Electric arc furnace
An electric arc furnace is a furnace that heats charged material by means of an electric arc.Arc furnaces range in size from small units of approximately one ton capacity up to about 400 ton units used for secondary steelmaking...
.
Technically perhaps, the first primitive open hearth furnace was the Catalan forge
Bloomery
A bloomery is a type of furnace once widely used for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. A bloomery's product is a porous mass of iron and slag called a bloom. This mix of slag and iron in the bloom is termed sponge iron, which...
, invented in Spain in the eighth century, but it is usual to confine the term to certain nineteenth century and later steelmaking processes, thus excluding bloomeries
Bloomery
A bloomery is a type of furnace once widely used for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. A bloomery's product is a porous mass of iron and slag called a bloom. This mix of slag and iron in the bloom is termed sponge iron, which...
(including the Catalan forge), finery forge
Finery forge
Iron tapped from the blast furnace is pig iron, and contains significant amounts of carbon and silicon. To produce malleable wrought iron, it needs to undergo a further process. In the early modern period, this was carried out in a finery forge....
s, and puddling furnaces from its application.
The Siemens regenerative furnace
Sir Carl Wilhelm SiemensCarl Wilhelm Siemens
Carl Wilhelm Siemens was a German born engineer who for most of his life worked in Britain and later became a British subject.-Biography:...
developed the Siemens regenerative furnace in the 1850s, and claimed in 1857 to be recovering enough heat to save 70–80% of the fuel. This furnace operates at a high temperature by using regenerative preheating
Air preheater
An air preheater is a general term to describe any device designed to heat air before another process with the primary objective of increasing the thermal efficiency of the process...
of fuel and air for combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. In regenerative preheating, the exhaust gases from the furnace are pumped into a chamber containing bricks, where heat is transferred from the gases to the bricks. The flow of the furnace is then reversed so that fuel and air pass through the chamber and are heated by the bricks. Through this method, an open-hearth furnace can reach temperatures high enough to melt steel, but Siemens did not initially use it for that.
The regenerators are the distinctive feature of the furnace and consist of fire-brick flues filled with bricks set on edge and arranged in such a way as to have a great number of small passages between them. The bricks absorb most of the heat from the outgoing waste gases and return it later to the incoming cold gases for combustion.
Open hearth steelmaking

Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
. Their process was known as the Siemens-Martin process, and the furnace as an "open-hearth" furnace. The most appealing characteristic of the Siemens regenerative furnace is the rapid production of large quantities of basic steel, used for example to construct high-rise buildings. The usual size of furnaces is 50 to 100 tons, but for some special processes they may have a capacity of 250 or even 500 tons. The Siemens-Martin process complemented rather than replaced the Bessemer process
Bessemer process
The Bessemer process was the first inexpensive industrial process for the mass-production of steel from molten pig iron. The process is named after its inventor, Henry Bessemer, who took out a patent on the process in 1855. The process was independently discovered in 1851 by William Kelly...
. It is slower and thus easier to control. It also permits the melting and refining of large amounts of scrap steel, further lowering steel production costs and recycling an otherwise troublesome waste material. Its worst drawback is the fact that melting and refining a charge takes several hours. This was an advantage in the early 20th C.,as it gave plant chemists time to analyze the steel and decide how much longer to refine it. But by about 1975, electronic instruments such as atomic absorption spectrophotometers had made analysis of the steel much easier and faster. The work environment around an open hearth furnace is said to be extremely dangerous, although that well be even more true of the environment around a basic oxygen or electric arc furnace.
The basic oxygen steelmaking
Basic oxygen steelmaking
Basic oxygen steelmaking , also known as Linz-Donawitz-Verfahren steelmaking or the oxygen converter process is a method of primary steelmaking in which carbon-rich molten pig iron is made into steel. Blowing oxygen through molten pig iron lowers the carbon content of the alloy and changes it into...
eventually replaced the open hearth furnace. It rapidly superseded both the Bessemer process and Siemens-Martin process in the Western Europe by the 1950s and in the Eastern Europe by the 1980s, the last European open hearth furnace being stopped in 1993. In the US, steel production using the open hearth furnaces had stopped by 1992. The last open hearth shop in China was shut down in 2001. The nation with the highest share of steel produced with open hearth furnaces (almost 50%) remains Ukraine. The process is still in use also in India and Russia.
Further reading
- K. Barraclough, Steelmaking 1850–1900 (Institute of Metals, London 1990), 137–203.
- W. K. V. Gale, Iron and Steel (Longmans, London 1969), 74–77.
External links
- Precursors to the Blast Furnace
- "Administering Doses of Liquid Iron to Steel Furnaces", Popular SciencePopular SciencePopular Science is an American monthly magazine founded in 1872 carrying articles for the general reader on science and technology subjects. Popular Science has won over 58 awards, including the ASME awards for its journalistic excellence in both 2003 and 2004...
, February 1919, page 64, scanned by Google Books.

