
Nitrone
Encyclopedia
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
of an imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
and a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
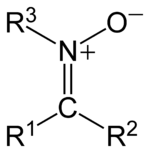
. The general structure is R1R2C=NR3+O- where R3 is different from H.
A nitrone is 1,3-dipole
1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms...
in 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
s. It reacts with alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s to form an isoxazolidine:
One example of this reaction type is the reaction of various Baylis-Hillman adducts with C-Phenyl-N-methylnitrone forming an isoxazolidine in which R1 is phenyl, R2 is hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and R3 is a methyl group .
Nitrones react with terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s and a copper salt to beta-lactam
Beta-lactam
A β-lactam ring, is a four-membered lactam. It is named as such, because the nitrogen atom is attached to the β-carbon relative to the carbonyl...
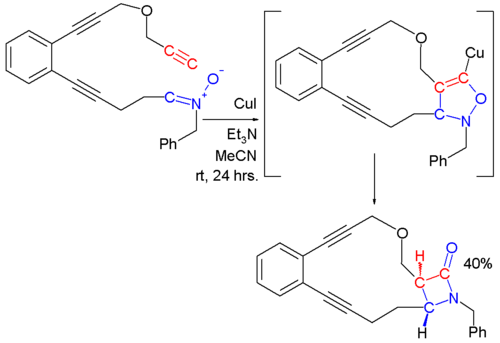
. This reaction is also called The Kinugasa reaction for example in this reaction::
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
generated copper(I) acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
to a 5-membered ring structure which rearranges in the second step.


