Nitroaldol reaction
Encyclopedia
The Henry Reaction is a classic carbon–carbon bond formation reaction in organic chemistry
. Discovered in 1895 by L. Henry, it is the combination of a nitroalkane and an aldehyde
or ketone
in the presence of a base to form β-Nitro alcohols. This type of reaction is commonly referred to as a "nitro-aldol" reaction (nitroalkane, aldehyde, and alcohol) It is nearly analogous to the aldol reaction
that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
Many of these uses have been exemplified in the syntheses of various pharmaceuticals including the β-blocker (S)-propranolol, the HIV protease inhibitor Amprenavir (Vertex 478), and construction of the carbohydrate subunit of the anthracycline class of antibiotics, L-Acosamine. The synthetic scheme of the L-Acosamine synthesis can be found in the Examples section of this article.
It is important to note that all steps of the Henry reaction are reversible. This is due to the lack of a committed step in the reaction to form product. It is for this reason that research has been geared towards modifications to drive the reaction to completion. More information about this can be found in the modification section of this article.
by orienting the nitro group and carbonyl oxygen anti (on opposite sides) each other. The R groups play a role in the transition state of the Henry reaction in that the larger the R groups are on each of the substrates, the more they will want to orient themselves away from each other (commonly referred to as steric effects
)
Due to a number of factors, including the reversibility of the reaction, as well as the tendency for easy epimerization of the nitro-substituted carbon atom, the Henry Reaction will typically produce a mixture of enantiomers or diastereomers. It is for this reason that explanations for stereoselectivity
remain scarce without some modification. In recent years, research focus has shifted toward modifications of the Henry Reaction to overcome this synthetic challenge.
One of the most frequently employed ways to induce enantio- or diastereoselectivity in the Henry Reaction has been through the use of chiral metal catalysts in which the nitro group and carbonyl oxygen coordinate to a metal that is bound to a chiral organic molecule. Some examples of metals that have been used include Zn, Co, Cu, Mg, and Cr. A depiction of this coordination is illustrated above.
The aza-Henry reaction is also used to produce nitroamines and can be a reliable synthetic route for the synthesis of vicinal diamines.
Perhaps one of the most synthetically useful modifications to the Henry Reaction is the use of an organocatalyst. The catalytic cycle is shown below.
List described that while this is a broad explanation, his brief review illustrates that this is a plausible mechanistic explanation for almost all reactions that involve an organocatalyst. An example of this type of reaction is illustrated in the Examples section of this article.
In addition to the previously mentioned modifications to the Henry reaction there are a variety of others. This includes the conversion of unreactive alkyl nitro compounds to their corresponding dianions which will react faster with carbonyl substrates, reactions can be accelerated using PAP as base, utilization of the reactivity of aldehydes with α,α-doubly deprotonated nitroalkanes to give nitronate alkoxides that yield mainly syn-nitro alcohols once protonated, and finally generation of nitronate anions in which one oxygenatom on the nitro group is silyl-protected to yield anti-β-nitro alcohols in the presence of a fluoride anion source when reacted with an aldehyde.
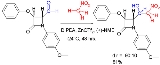
Industrial Application- An enantioselective aldol addition product can be obtained in asymmetric synthesis by reaction of benzaldehyde with nitromethane
and the a catalyst system consisting of a zinc
triflate salt / the base diisopropylethylamine (DIPEA) and as chiral ligand
is the N-methyl derivative of (+)-ephedrine
(NME).
A diastereoselective variation of this reaction is depicted below.
 Total Synthesis- In 2005, Barua and coworkers completed the total synthesis of the potent aminopeptidase inhibitor, (-)-bestatin, in an overall yield of 26% overall yield employing Shisasaki's asymmetric Henry reaction as the key step. (illustrated below)
Total Synthesis- In 2005, Barua and coworkers completed the total synthesis of the potent aminopeptidase inhibitor, (-)-bestatin, in an overall yield of 26% overall yield employing Shisasaki's asymmetric Henry reaction as the key step. (illustrated below)
Organocatalysis- In 2006, Hiemstra and coworkers explored the use of Cinchona derivatives as asymmetric catalysts for the reaction between aromatic aldehydes and nitromethane. Through the use of particular derivatives, they were able to induce direct enantioselection through the use of the proper catalyst.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Discovered in 1895 by L. Henry, it is the combination of a nitroalkane and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
in the presence of a base to form β-Nitro alcohols. This type of reaction is commonly referred to as a "nitro-aldol" reaction (nitroalkane, aldehyde, and alcohol) It is nearly analogous to the aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
Many of these uses have been exemplified in the syntheses of various pharmaceuticals including the β-blocker (S)-propranolol, the HIV protease inhibitor Amprenavir (Vertex 478), and construction of the carbohydrate subunit of the anthracycline class of antibiotics, L-Acosamine. The synthetic scheme of the L-Acosamine synthesis can be found in the Examples section of this article.
Mechanism
The Henry reaction begins with the deprotonation of the nitroalkane on the α-carbon position forming a resonance stabilized anion. This is followed by alkylation of the nitroalkane with the carbonyl containing substrate to form a diastereomeric β-nitro alkoxide. The protonation of the alkoxide by the previously protonated base will yield the respective β-nitro alcohol as product. (Scheme below)It is important to note that all steps of the Henry reaction are reversible. This is due to the lack of a committed step in the reaction to form product. It is for this reason that research has been geared towards modifications to drive the reaction to completion. More information about this can be found in the modification section of this article.
Stereochemical Course
One of the commonly accepted models for stereoselection without any modification to the Henry reaction is shown below where stereoselectivity is governed by the size of the R groups in the model (ex. carbon chain) as well as a transition state that minimizes dipoleDipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
by orienting the nitro group and carbonyl oxygen anti (on opposite sides) each other. The R groups play a role in the transition state of the Henry reaction in that the larger the R groups are on each of the substrates, the more they will want to orient themselves away from each other (commonly referred to as steric effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
)
Due to a number of factors, including the reversibility of the reaction, as well as the tendency for easy epimerization of the nitro-substituted carbon atom, the Henry Reaction will typically produce a mixture of enantiomers or diastereomers. It is for this reason that explanations for stereoselectivity
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
remain scarce without some modification. In recent years, research focus has shifted toward modifications of the Henry Reaction to overcome this synthetic challenge.
One of the most frequently employed ways to induce enantio- or diastereoselectivity in the Henry Reaction has been through the use of chiral metal catalysts in which the nitro group and carbonyl oxygen coordinate to a metal that is bound to a chiral organic molecule. Some examples of metals that have been used include Zn, Co, Cu, Mg, and Cr. A depiction of this coordination is illustrated above.
General Features
One of the many features of the Henry Reaction that makes it synthetically attractive is that it utilizes only a catalytic amount of base to drive the reaction. Additionally a variety of bases can be used including ionic bases such as alkali metal hydroxides, alkoxides, carbonates, and sources of fluoride anion (e.g. TBAF) or nonionic organic amine bases including TMG, DBU, DBN, and PAP. It is important to note that the base and solvent used do not have a large influence on the overall outcome of the reaction.Limitations
One of the main drawbacks of the Henry Reaction is the potential for side reactions throughout the course of the reaction. Aside from the reversibility of the reaction (Retro-Henry) which could prevent the reaction from proceeding, the β-nitro alcohol has the potential to undergo dehydration, and for sterically hindered substrates it is possible that a base catalyzed self-condensation (Cannizaro reaction) could occur. A general scheme of the Cannizzaro reaction is depicted below.Modifications
There have been a series of modifications made to the Henry Reaction. Of these some of the most important include employing high-pressure and sometimes solvent free conditions to improve chemo- and regioselectivity and chiral metal catalysts to induce enantio-or diastereoselectivity.The aza-Henry reaction is also used to produce nitroamines and can be a reliable synthetic route for the synthesis of vicinal diamines.
Perhaps one of the most synthetically useful modifications to the Henry Reaction is the use of an organocatalyst. The catalytic cycle is shown below.
List described that while this is a broad explanation, his brief review illustrates that this is a plausible mechanistic explanation for almost all reactions that involve an organocatalyst. An example of this type of reaction is illustrated in the Examples section of this article.
In addition to the previously mentioned modifications to the Henry reaction there are a variety of others. This includes the conversion of unreactive alkyl nitro compounds to their corresponding dianions which will react faster with carbonyl substrates, reactions can be accelerated using PAP as base, utilization of the reactivity of aldehydes with α,α-doubly deprotonated nitroalkanes to give nitronate alkoxides that yield mainly syn-nitro alcohols once protonated, and finally generation of nitronate anions in which one oxygenatom on the nitro group is silyl-protected to yield anti-β-nitro alcohols in the presence of a fluoride anion source when reacted with an aldehyde.
Examples
Industrial Application- In 1999, Menzel and coworkers developed a synthetic route to obtaining L-Acosamine, the carbohydrate subunit of the anthracycline class of antibioticsIndustrial Application- An enantioselective aldol addition product can be obtained in asymmetric synthesis by reaction of benzaldehyde with nitromethane
Nitromethane
Nitromethane is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a slightly viscous, highly polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent...
and the a catalyst system consisting of a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
triflate salt / the base diisopropylethylamine (DIPEA) and as chiral ligand
Chiral ligand
In chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the...
is the N-methyl derivative of (+)-ephedrine
Ephedrine
Ephedrine is a sympathomimetic amine commonly used as a stimulant, appetite suppressant, concentration aid, decongestant, and to treat hypotension associated with anaesthesia....
(NME).
A diastereoselective variation of this reaction is depicted below.

Organocatalysis- In 2006, Hiemstra and coworkers explored the use of Cinchona derivatives as asymmetric catalysts for the reaction between aromatic aldehydes and nitromethane. Through the use of particular derivatives, they were able to induce direct enantioselection through the use of the proper catalyst.

