_chloride.gif)
Niobium(V) chloride
Encyclopedia
Niobium chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium
. NbCl5 may be purified by sublimation
.
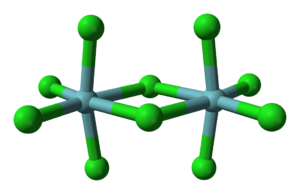 Niobium(V) chloride forms chloro-bridged dimers in the solid state (see figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond length
Niobium(V) chloride forms chloro-bridged dimers in the solid state (see figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond length
s are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, TaCl5
and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures.
In the laboratory, niobium pentachloride is often prepared from Nb2O5, the main problem being incomplete reaction to give the oxyhalides. The conversion can be effected with thionyl chloride
: It also can be prepared by chlorination of niobium pentoxide
in the presence of carbon at 300°C. The products, however, contain small amounts of NbOCl3
.
In organic synthesis
, NbCl3 is a specialized Lewis acid
in activating alkene
s for the carbonyl-ene reaction and the Diels-Alder reaction. Niobium chloride can also generate N-acyliminium compounds from certain pyrrolidine
s which are substrates for nucleophile
s such as allyltrimethylsilane, indole
, or the silyl enol ether
of benzophenone
.
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
. NbCl5 may be purified by sublimation
Sublimation (physics)
Sublimation is the process of transition of a substance from the solid phase to the gas phase without passing through an intermediate liquid phase...
.
Structure and properties

Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, TaCl5
Tantalum(V) chloride
Tantalum chloride, also known as tantalum pentachloride, is the inorganic compound with the formula TaCl5. This white powder is a starting material in tantalum chemistry. It hydrolyzes readily, releasing HCl. TaCl5 is prepared by heating tantalum metal in chlorine...
and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures.
Preparation
Industrially, niobium pentachloride is obtained by direct chlorination of niobium metal at 300 to 350 °C:- 2 Nb + 5 Cl2 → 2 NbCl5
In the laboratory, niobium pentachloride is often prepared from Nb2O5, the main problem being incomplete reaction to give the oxyhalides. The conversion can be effected with thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
: It also can be prepared by chlorination of niobium pentoxide
Niobium pentoxide
Niobium pentoxide is the inorganic compound with the formula Nb2O5. It is a colourless insoluble solid that is fairly unreactive. It is the main precursor to all materials made of niobium, the dominant application being alloys, but other specialized applications include capacitors, lithium niobate,...
in the presence of carbon at 300°C. The products, however, contain small amounts of NbOCl3
Niobium oxychloride
Niobium oxychloride is the inorganic compound with the formula NbOCl3. It is a white, crystalline, diamagnetic solid. It is often found as an impurity in samples of niobium pentachloride, a common reagent in niobium chemistry.- Structure and synthesis :...
.
Uses
Niobium(V) chloride is the main precursor to the alkoxides of niobium, which find niche uses in sol-gel processing. It is also the precursor to many other laboratory reagents.In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, NbCl3 is a specialized Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
in activating alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s for the carbonyl-ene reaction and the Diels-Alder reaction. Niobium chloride can also generate N-acyliminium compounds from certain pyrrolidine
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom...
s which are substrates for nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s such as allyltrimethylsilane, indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
, or the silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
of benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
.

