
Multiple isomorphous replacement
Encyclopedia
Multiple isomorphous replacement or MIR is the most common approach of solving the phase problem
in X-ray crystallography
. This method is conducted by soaking the crystal of a sample to be analyzed with a heavy atom solution or co-crystallization with the heavy atom. The addition of the heavy atom should not affect the crystal formation or unit cell dimensions in comparison to its native form, hence, they should be isomorphic
.
Data sets from the native and heavy-atom derivative of the sample are first collected. Then the interpretation of the Patterson difference map
reveals the heavy atom's location in the unit cell. This allows both the amplitude
and the phase
of the atom to be determined. Since the structure factor
of the heavy atom derivative (Fph) of the crystal is the vector sum of the lone heavy atom (Fh) and the native crystal (Fp) then the phase of the native Fp and Fph vectors can be solved geometrically.
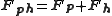
At least two isomorphous derivatives must be evaluated since using only one will give two possible phases.
Phase problem
In physics the phase problem is the name given to the problem of loss of information concerning the phase that can occur when making a physical measurement. The name itself comes from the field of x-ray crystallography, where the phase problem has to be solved for the determination of a structure...
in X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. This method is conducted by soaking the crystal of a sample to be analyzed with a heavy atom solution or co-crystallization with the heavy atom. The addition of the heavy atom should not affect the crystal formation or unit cell dimensions in comparison to its native form, hence, they should be isomorphic
Isomorphism (crystallography)
In crystallography crystals are described as isomorphous if they are closely similar in shape. Historically crystal shape was defined by measuring the angles between crystal faces with a goniometer...
.
Data sets from the native and heavy-atom derivative of the sample are first collected. Then the interpretation of the Patterson difference map
Patterson function
The Patterson function is used to solve the phase problem in X-ray crystallography. It was introduced in 1935 by Arthur Lindo Patterson while he was a visiting researcher in the laboratory of Bertram Eugene Warren at MIT....
reveals the heavy atom's location in the unit cell. This allows both the amplitude
Amplitude
Amplitude is the magnitude of change in the oscillating variable with each oscillation within an oscillating system. For example, sound waves in air are oscillations in atmospheric pressure and their amplitudes are proportional to the change in pressure during one oscillation...
and the phase
Phase (waves)
Phase in waves is the fraction of a wave cycle which has elapsed relative to an arbitrary point.-Formula:The phase of an oscillation or wave refers to a sinusoidal function such as the following:...
of the atom to be determined. Since the structure factor
Structure factor
In condensed matter physics and crystallography, the static structure factor is a mathematical description of how a material scatters incident radiation...
of the heavy atom derivative (Fph) of the crystal is the vector sum of the lone heavy atom (Fh) and the native crystal (Fp) then the phase of the native Fp and Fph vectors can be solved geometrically.
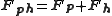
At least two isomorphous derivatives must be evaluated since using only one will give two possible phases.
Examples
Some examples of heavy atoms used in protein MIR:- HgMercury (element)Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
2+ ions bind to thiolThiolIn organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
groups. - UranylUranylThe uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula [UO2]2+. It has a linear structure with short U-O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands are bound to the uranyl ion in an equatorial plane...
salts (UO2Uranium dioxideUranium dioxide or uranium oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used...
+ NO3NitrateThe nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
) bind between carboxyl groups in AspAspartic acidAspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
and GluGlutamic acidGlutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates... - PbLeadLead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
bind to CysCysteineCysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid... - PtCl42-Platinum(IV) chloridePlatinum chloride is the inorganic compound of platinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state.-Structure:...
(ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
) bind to HisHistidineHistidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
Anomalous Dispersion
- Multi-wavelength anomalous dispersionMulti-wavelength anomalous dispersionMulti-wavelength anomalous diffraction is a technique used in X-ray crystallography that facilitates the determination of the three-dimensional structure of biological macromolecules via solution of the phase problem...
(MAD) - Single wavelength anomalous dispersionSingle wavelength anomalous dispersionSingle-wavelength anomalous dispersion is a technique used in X-ray crystallography that facilitates the determination of the structure of proteins or other biological macromolecules by allowing the solution of the phase problem. In contrast to multi-wavelength anomalous dispersion, SAD uses a...
(SAD)
Isomorphous Replacement
Two methods for providing the needed phasing information by introducing heavy atoms into isomorphous crystals:- Multiple isomorphous replacement (MIR); and
- Single isomorphous replacement with anomalous signal (SIRAS)
External links
- MAD phasing — an in depth tutorial with examples, illustrations, and references.
Computer programs
- The SSRL Absorption Package —
- CHOOCH —
- Shake-and-Bake (SnB) —
- SHELX —

