
Lower critical solution temperature
Encyclopedia
The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible for all compositions. The word lower indicates that the LCST is a lower bound to a temperature interval of partial miscibility, or miscibility for certain compositions only.
The phase behavior of polymer solutions is an important property involved in the development and design of most polymer-related processes. Partially miscible polymer solutions often exhibit two solubility boundaries, the upper critical solution temperature (UCST) and the lower
critical solution temperature (LCST), which both depend on the molar mass and the pressure. At temperatures below LCST, the system is completely miscible in all proportions,whereas above LCST partial liquid miscibility occurs.
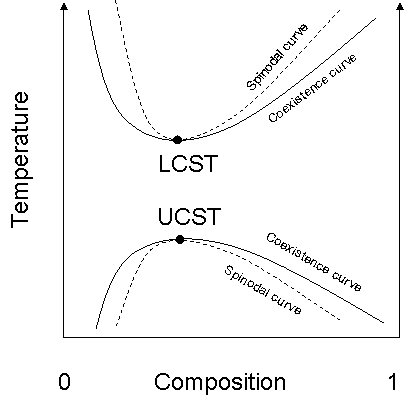
In the phase diagram
of the mixture components, the LCST is the shared minimum of the concave up spinodal
and binodal
(or coexistence) curves. It is in general pressure
dependent, increasing as a function of increased pressure.
For small molecules, the existence of an LCST is much less common than the existence of a upper critical solution temperature
(UCST), but some cases do exist. For example, the system triethylamine
-water has an LCST of 19°C, so that these two substances are miscible in all proportions below 19°C but not at higher temperatures. The nicotine
-water system has an LCST of 61°C, and also a UCST of 210°C at pressures high enough for liquid water to exist at that temperature. The components are therefore miscible in all proportions below 61°C and above 210°C (at high pressure), and partially miscible in the interval from 61 to 210°C.
 Some polymer
Some polymer
solutions have an LCST at temperatures higher than the UCST. As shown in the diagram, this means that there is a temperature interval of complete miscibility, with partial miscibility at both higher and lower temperatures.
In the case of polymer solutions, the LCST also depends on polymer degree of polymerization
, polydispersity
and branching as well as on the polymer's composition and architecture . A prominent polymer possessing an LCST is Poly(N-isopropylacrylamide)
in water, which undergoes a reversible collapse transition related to the LCST at 33°C.
The LCST depends on the polymer preparation and in the case of copolymers, the monomer ratios, as well as the hydrophobic or hydrophilic nature of the polymer.
. Since mixing of the two phases is spontaneous below the LCST and not above, the Gibbs free energy
change (ΔG) for the mixing of these two phases is negative below the LCST and positive above, and the entropy change ΔS = – (dΔG/dT) is negative for this mixing process. This is in contrast to the more common and intuitive case in which entropies drive mixing due to the increased volume accessible to each component upon mixing.
In general, the unfavorable entropy of mixing responsible for the LCST has one of two physical origins. The first is associating interactions between the two components such as strong polar interactions or hydrogen bond
s, which prevent random mixing. For example in the triethylamine-water system, the amine molecules cannot form hydrogen bonds with each other but only with water molecules, so in solution they remain associated to water molecules with loss of entropy. The mixing which occurs below 19oC is due not to entropy but to the enthalpy of formation of the hydrogen bonds.
The second physical factor which can lead to an LCST is compressibility effects, especially in polymer-solvent systems. For nonpolar systems such as polystyrene
in cyclohexane
, phase separation has been observed in sealed tubes (at high pressure) at temperatures approaching the liquid-vapor critical point
of the solvent. At such temperatures the solvent expands much more rapidly than the polymer, whose segments are covalently linked. Mixing therefore requires contraction of the solvent for compatibility of the polymer, resulting in a loss of entropy.
, the LCST may be modeled theoretically via the lattice fluid model, an extension of Flory–Huggins solution theory, that incorporates vacancies, and thus accounts for variable density and compressibility effects.
The phase behavior of polymer solutions is an important property involved in the development and design of most polymer-related processes. Partially miscible polymer solutions often exhibit two solubility boundaries, the upper critical solution temperature (UCST) and the lower
critical solution temperature (LCST), which both depend on the molar mass and the pressure. At temperatures below LCST, the system is completely miscible in all proportions,whereas above LCST partial liquid miscibility occurs.
In the phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
of the mixture components, the LCST is the shared minimum of the concave up spinodal
Spinodal
In thermodynamics, the spinodal is the limit of stability of a solution, denoting the boundary of absolute instability of a solution to decomposition into multiple phases. Within this curve, infinitesimally small fluctuations in composition and density will lead to phase separation via spinodal...
and binodal
Binodal
In thermodynamics, the binodal, also known as the coexistence curve or binodal curve, denotes the condition at which two distinct phases may coexist...
(or coexistence) curves. It is in general pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
dependent, increasing as a function of increased pressure.
For small molecules, the existence of an LCST is much less common than the existence of a upper critical solution temperature
Upper critical solution temperature
The upper critical solution temperature or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions. The word upper indicates that the UCST is an upper bound to a temperature range of partial miscibility, or miscibility for...
(UCST), but some cases do exist. For example, the system triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
-water has an LCST of 19°C, so that these two substances are miscible in all proportions below 19°C but not at higher temperatures. The nicotine
Nicotine
Nicotine is an alkaloid found in the nightshade family of plants that constitutes approximately 0.6–3.0% of the dry weight of tobacco, with biosynthesis taking place in the roots and accumulation occurring in the leaves...
-water system has an LCST of 61°C, and also a UCST of 210°C at pressures high enough for liquid water to exist at that temperature. The components are therefore miscible in all proportions below 61°C and above 210°C (at high pressure), and partially miscible in the interval from 61 to 210°C.
Polymer-solvent mixtures

Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
solutions have an LCST at temperatures higher than the UCST. As shown in the diagram, this means that there is a temperature interval of complete miscibility, with partial miscibility at both higher and lower temperatures.
In the case of polymer solutions, the LCST also depends on polymer degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
, polydispersity
Polydispersity index
In physical and organic chemistry, the polydispersity index , is a measure of the distribution of molecular mass in a given polymer sample. The PDI calculated is the weight average molecular weight divided by the number average molecular weight. It indicates the distribution of individual...
and branching as well as on the polymer's composition and architecture . A prominent polymer possessing an LCST is Poly(N-isopropylacrylamide)
Poly(N-isopropylacrylamide)
Poly is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from NIPAM which is commercially available....
in water, which undergoes a reversible collapse transition related to the LCST at 33°C.
The LCST depends on the polymer preparation and in the case of copolymers, the monomer ratios, as well as the hydrophobic or hydrophilic nature of the polymer.
Physical basis
A key physical factor which distinguishes the LCST from other mixture behavior is that the LCST phase separation is driven by unfavorable entropy of mixingEntropy of mixing
In thermodynamics the entropy of mixing is the increase in the total entropy of a compound system, when different and chemically non-reacting chemical substances or material components are mixed by removing partition between the system's initially separate volumes...
. Since mixing of the two phases is spontaneous below the LCST and not above, the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
change (ΔG) for the mixing of these two phases is negative below the LCST and positive above, and the entropy change ΔS = – (dΔG/dT) is negative for this mixing process. This is in contrast to the more common and intuitive case in which entropies drive mixing due to the increased volume accessible to each component upon mixing.
In general, the unfavorable entropy of mixing responsible for the LCST has one of two physical origins. The first is associating interactions between the two components such as strong polar interactions or hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, which prevent random mixing. For example in the triethylamine-water system, the amine molecules cannot form hydrogen bonds with each other but only with water molecules, so in solution they remain associated to water molecules with loss of entropy. The mixing which occurs below 19oC is due not to entropy but to the enthalpy of formation of the hydrogen bonds.
The second physical factor which can lead to an LCST is compressibility effects, especially in polymer-solvent systems. For nonpolar systems such as polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
in cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
, phase separation has been observed in sealed tubes (at high pressure) at temperatures approaching the liquid-vapor critical point
Critical point (thermodynamics)
In physical chemistry, thermodynamics, chemistry and condensed matter physics, a critical point, also called a critical state, specifies the conditions at which a phase boundary ceases to exist...
of the solvent. At such temperatures the solvent expands much more rapidly than the polymer, whose segments are covalently linked. Mixing therefore requires contraction of the solvent for compatibility of the polymer, resulting in a loss of entropy.
Theory
Within statistical mechanicsStatistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
, the LCST may be modeled theoretically via the lattice fluid model, an extension of Flory–Huggins solution theory, that incorporates vacancies, and thus accounts for variable density and compressibility effects.

