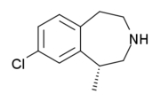
Lorcaserin
Encyclopedia
Lorcaserin is a weight-loss drug developed by Arena Pharmaceuticals
. It has serotonergic
properties and acts as an anorectic
. On 22 December 2009 a New Drug Application
(NDA) was submitted to the Food and Drug Administration
(FDA) in the United States
and lorcaserin is pending approval . On 16 September, an FDA advisory panel voted to recommend against approval of the drug based on concerns over both safety and efficacy. In October 2010, the FDA stated that it that it could not approve the application for lorcaserin in its present form.
agonist
, and in vitro testing of the drug showed reasonable selectivity for 5-HT2C over other related targets. 5-HT2C receptors are located almost exclusively in the brain, and can be found in the choroid plexus
, cortex
, hippocampus
, cerebellum
, amygdala
, thalamus
, and hypothalamus
. The activation of 5-HT2C receptors in the hypothalamus is supposed to activate proopiomelanocortin
(POMC) production and consequently promote weight loss through satiety. This hypothesis is supported by clinical trials and other studies. While it is generally thought that 5-HT2C receptors help to regulate appetite as well as mood, and endocrine secretion, the exact mechanism of appetite regulation is not yet known. Lorcaserin has shown 100:1 affinity for 5-HT2C versus other receptors.
group lost an average of 0.7 pounds, despite the fact that all groups received no diet or exercise instruction.
Upon discontinuation of lorcaserin treatment, lost weight is regained. In Phase 2 clinical trials, patients were tracked for 2 weeks post trial completion, and all groups regained weight more rapidly than they had lost. In pre-clinical trial studies, the weight of rats returned to control levels.
using an intention to treat – last observation carried forward (ITT-LOCF), analysis showed that 47.5% of lorcaserin patients lost at least 5% of their body weight, compared to 20.3% for placebo. This result satisfies one of two alternate efficacy benchmarks in the most recent FDA draft guidance, which provides that a weight-management product can be considered effective if after one year of treatment the proportion of subjects who lose greater than or equal to 5% of baseline body weight in the active-product group is at least 35%, is approximately double the proportion in the placebo-treated group, and the difference between groups is statistically significant.
Additionally, 22.6% of lorcaserin patients lost at least 10% of their body weight, compared to 7.7% for placebo. Lorcaserin patients achieved an average weight loss of 5.8% of their body weight, or 12.7 pounds, compared to 2.2%, or 4.7 pounds, for placebo. Among the most frequent adverse events reported with lorcaserin were headache, dizziness, and nausea.
. It was scheduled to end in May 2010. , no results are available.
(PDUFA) date of 22 October 2010. On 16 September 2010, a federal advisory committee voted against recommending approval for lorcaserin. In their 9-5 vote, the committee had raised concerns about the safety of the drug, particularly the findings of tumors in rats.
On 23 October 2010, the FDA decided not to approve the drug based on the available data. This was not only because of cancer promoting properties could not be ruled out, but also because the weight loss efficacy was "marginal".
(18% vs 9% for placebo), orlistat
(7% vs 4% for placebo), sibutramine
(9% vs 9%) and rimonabant
(15% vs 7%).
Lorcaserin produced side effects in human clinical trials, but at rates not significantly different than placebo and mostly with mild and transient severity. The most common side effect was headache
, experienced by about 18% of drug arm participants compared to 11% of placebo participants. Headache was the only reported side effect to occur at a frequency greater than 5 percentage points above placebo. Other reported side effects and their rates for lorcaserin and placebo patients, respectively, were as follows: upper respiratory tract infection
(14.8% vs. 11.9%), nasopharyngitis (13.4% vs. 12.0%), sinusitis
(7.2% vs. 8.2%) and nausea
(7.5% vs. 5.4%). Adverse events of depression
, anxiety
and suicidal ideation
were infrequent and were reported at a similar rate in each treatment group.
On 15 September 2010 it was reported by national news-media that lorcaserin was associated with the development of cancer in laboratory rats.
, Arena Pharmaceuticals sought to rule out an increase in the rate of valvulopathy
of 20% or more because a number of anorectic drugs have been withdrawn for cardiovascular side-effects. In agreement with the FDA, Arena conducted regular and multiple echocardiograms of the phase III participants. At the 3, 6, and 12-month interval, the echocardiograms of participants of the BLOOM trial did not show any significant increase in valvulopathy over baseline, so the independent Echocardiographic Data Safety Monitoring Board (EDSMB) hired to monitor the trial allowed the trial to continue to the end. BLOOM participants received 18- and 24-month follow-up echocardiograms, but these results will not be reviewed by an EDSMB. The two other Phase 3 trials provide multiple and regular echocardiograms but they were not be reviewed by an EDSMB. BLOOM participants were pre-screened to exclude valvulopathy, but BLOSSOM and BLOOM-DM participants were not. Like BLOOM, BLOSSOM showed no significant increase in valvulopathy.
Rates of new FDA-defined valvulopathy in BLOOM were as follows: lorcaserin 10 mg twice daily (2.7%) and placebo (2.3%) at Week 52 and lorcaserin 10 mg twice daily (2.6%) and placebo (2.7%) at Week 104. For BLOSSOM, rates of new FDA-defined valvulopathy in BLOSSOM at Week 52 were as follows: lorcaserin 10 mg twice daily (2.0%), 10 mg once daily (1.4%) and placebo (2.0%).
with a structure similar to dexfenfluramine
, an anorectic drug that was withdrawn because of cardiovascular side-effects.
has forecast lorcaserin sales of $3 billion in 2015. Arena has a very close financial relationship a group of private funds under the Deerfield umbrella name (Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P., Deerfield Partners, L.P., Deerfield International Limited, Deerfield Special Situations Fund, L.P., and Deerfield Special Situations Fund International Limited) under which Deerfield has lent Arena $100 million, purchased 11 million shares, and holds 28 million warrants to purchase additional shares at prices ranging from $3.23 to $5.48. On 15 September 2010 shares of Arena fell nearly 40%, from $6.85 to $4.13, on news that the lorcaserin has been linked to formation of malignant tumors in rats. On 16 September, an FDA advisory panel voted 9 to 5 to recommend against approval of the drug based on concerns over both safety and efficacy, trading on Arena's stock was stopped on that date, but after hours, the stock price fell about 40 percent.
Arena Pharmaceuticals
Arena Pharmaceuticals, Inc. is a biopharmaceutical company located in San Diego, California. The company is developing oral medications that target G-protein-coupled receptors. Currently none of the drugs is approved by the U.S...
. It has serotonergic
Serotonergic
Serotonergic or serotoninergic means "related to the neurotransmitter serotonin". A synapse is serotonergic if it uses serotonin as its neurotransmitter...
properties and acts as an anorectic
Anorectic
An anorectic or anorexic , also known as anorexigenic or appetite suppressant, is a dietary supplement and/or drug which reduces appetite, food consumption, and as a result, causes weight loss to occur.-List of anorectics:Numerous pharmaceutical compounds are marketed as appetite suppressants.The...
. On 22 December 2009 a New Drug Application
New drug application
The New Drug Application is the vehicle in the United States through which drug sponsors formally propose that the Food and Drug Administration approve a new pharmaceutical for sale and marketing...
(NDA) was submitted to the Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA) in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
and lorcaserin is pending approval . On 16 September, an FDA advisory panel voted to recommend against approval of the drug based on concerns over both safety and efficacy. In October 2010, the FDA stated that it that it could not approve the application for lorcaserin in its present form.
Mechanism of action
Lorcaserin is a selective 5-HT2C receptor5-HT2C receptor
The 5-HT2C receptor is a subtype of 5-HT receptor that binds the endogenous neurotransmitter serotonin . It is a G protein-coupled receptor that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located at the...
agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
, and in vitro testing of the drug showed reasonable selectivity for 5-HT2C over other related targets. 5-HT2C receptors are located almost exclusively in the brain, and can be found in the choroid plexus
Choroid plexus
The choroid plexus is a structure in the ventricles of the brain where cerebrospinal fluid is produced...
, cortex
Cerebral cortex
The cerebral cortex is a sheet of neural tissue that is outermost to the cerebrum of the mammalian brain. It plays a key role in memory, attention, perceptual awareness, thought, language, and consciousness. It is constituted of up to six horizontal layers, each of which has a different...
, hippocampus
Hippocampus
The hippocampus is a major component of the brains of humans and other vertebrates. It belongs to the limbic system and plays important roles in the consolidation of information from short-term memory to long-term memory and spatial navigation. Humans and other mammals have two hippocampi, one in...
, cerebellum
Cerebellum
The cerebellum is a region of the brain that plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language, and in regulating fear and pleasure responses, but its movement-related functions are the most solidly established...
, amygdala
Amygdala
The ' are almond-shaped groups of nuclei located deep within the medial temporal lobes of the brain in complex vertebrates, including humans. Shown in research to perform a primary role in the processing and memory of emotional reactions, the amygdalae are considered part of the limbic system.-...
, thalamus
Thalamus
The thalamus is a midline paired symmetrical structure within the brains of vertebrates, including humans. It is situated between the cerebral cortex and midbrain, both in terms of location and neurological connections...
, and hypothalamus
Hypothalamus
The Hypothalamus is a portion of the brain that contains a number of small nuclei with a variety of functions...
. The activation of 5-HT2C receptors in the hypothalamus is supposed to activate proopiomelanocortin
Proopiomelanocortin
Pro-opiomelanocortin is a precursor polypeptide with 241 amino acid residues. POMC is synthesized from the 285-amino acid long polypeptide precursor, pre-pro-opiomelanocortin , by the removal of a 44-amino acid long signal peptide sequence during translation.The POMC gene is located on chromosome...
(POMC) production and consequently promote weight loss through satiety. This hypothesis is supported by clinical trials and other studies. While it is generally thought that 5-HT2C receptors help to regulate appetite as well as mood, and endocrine secretion, the exact mechanism of appetite regulation is not yet known. Lorcaserin has shown 100:1 affinity for 5-HT2C versus other receptors.
Phase IIb and other early clinical trial results
Arena states that "[d]ata from Phase 2 clinical trials of lorcaserin demonstrated that patients who received the drug experienced significantly greater weight loss than patients who received placebo." At the end of 12 weeks, the groups which were administered lorcaserin lost an average of 4.0 pounds (10 mg/day), 5.7 pounds (15 mg/day), and 7.9 pounds (20 mg/day). The placeboPlacebo
A placebo is a simulated or otherwise medically ineffectual treatment for a disease or other medical condition intended to deceive the recipient...
group lost an average of 0.7 pounds, despite the fact that all groups received no diet or exercise instruction.
Upon discontinuation of lorcaserin treatment, lost weight is regained. In Phase 2 clinical trials, patients were tracked for 2 weeks post trial completion, and all groups regained weight more rapidly than they had lost. In pre-clinical trial studies, the weight of rats returned to control levels.
Phase III clinical trials
The Lorcaserin Phase III program consists of three different Phase III trials, BLOSSOM (Behavioral modification and LOrcaserin Second Study for Obesity Management), BLOOM (Behavioral modification and Lorcaserin for Overweight and Obesity Management), and BLOOM-DM (Diabetes Management).BLOOM
BLOOM top line results were released on 30 March 2009. Measurements of efficacyEfficacy
Efficacy is the capacity to produce an effect. It has different specific meanings in different fields. In medicine, it is the ability of an intervention or drug to reproduce a desired effect in expert hands and under ideal circumstances.- Healthcare :...
using an intention to treat – last observation carried forward (ITT-LOCF), analysis showed that 47.5% of lorcaserin patients lost at least 5% of their body weight, compared to 20.3% for placebo. This result satisfies one of two alternate efficacy benchmarks in the most recent FDA draft guidance, which provides that a weight-management product can be considered effective if after one year of treatment the proportion of subjects who lose greater than or equal to 5% of baseline body weight in the active-product group is at least 35%, is approximately double the proportion in the placebo-treated group, and the difference between groups is statistically significant.
Additionally, 22.6% of lorcaserin patients lost at least 10% of their body weight, compared to 7.7% for placebo. Lorcaserin patients achieved an average weight loss of 5.8% of their body weight, or 12.7 pounds, compared to 2.2%, or 4.7 pounds, for placebo. Among the most frequent adverse events reported with lorcaserin were headache, dizziness, and nausea.
BLOSSOM
BLOSSOM results were released on September 18, 2009. Measurements of efficacy using an intent-to-treat last observation carried forward, or ITT-LOCF, analysis showed that 47.2% of lorcaserin patients lost at least 5% of their body weight, compared to 25.0% for placebo. Lorcaserin patients achieved an average weight loss of 5.9%, or 12.7 pounds, compared to 2.8%, or 6.3 pounds, for placebo.BLOOM-DM
This trial examined the effect of lorcaserin in patients with diabetes mellitusDiabetes mellitus
Diabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced...
. It was scheduled to end in May 2010. , no results are available.
Time schedule
Lorcaserin had a Prescription Drug User Fee ActPrescription Drug User Fee Act
The Prescription Drug User Fee Act was a law passed by the United States Congress in 1992 which allowed the Food and Drug Administration to collect fees from drug manufacturers to fund the new drug approval process...
(PDUFA) date of 22 October 2010. On 16 September 2010, a federal advisory committee voted against recommending approval for lorcaserin. In their 9-5 vote, the committee had raised concerns about the safety of the drug, particularly the findings of tumors in rats.
On 23 October 2010, the FDA decided not to approve the drug based on the available data. This was not only because of cancer promoting properties could not be ruled out, but also because the weight loss efficacy was "marginal".
Side effects
Generally, lorcaserin was shown in phase III testing to be a mild and tolerable agent. Trial participants found lorcaserin about as tolerable as placebo, with 7% of participants in both the lorcaserin drug and placebo arms dropping out due to side effects. Lorcaserin had the lowest discontinuation rates due to adverse events of any obesity drug in a phase III trial, as compared to discontinuation rates due to adverse events for bupropion/naltrexone (26% vs 13% for placebo), phentermine/topiramatePhentermine/topiramate
The combination of the drugs phentermine and topiramate is an investigational medication for the treatment of obesity and related conditions such as type 2 diabetes and has been found to lower blood pressure and cholesterol. Qnexa is being developed by Vivus, a California pharmaceutical company...
(18% vs 9% for placebo), orlistat
Orlistat
Orlistat , also known as tetrahydrolipstatin, is a drug designed to treat obesity. Its primary function is preventing the absorption of fats from the human diet, thereby reducing caloric intake...
(7% vs 4% for placebo), sibutramine
Sibutramine
Sibutramine is an oral anorexiant. Until 2010 it was marketed and prescribed as an adjunct in the treatment of exogenous obesity along with diet and exercise...
(9% vs 9%) and rimonabant
Rimonabant
Rimonabant is an anorectic antiobesity drug that has been withdrawn from the market. It is an inverse agonist for the cannabinoid receptor CB1...
(15% vs 7%).
Lorcaserin produced side effects in human clinical trials, but at rates not significantly different than placebo and mostly with mild and transient severity. The most common side effect was headache
Headache
A headache or cephalalgia is pain anywhere in the region of the head or neck. It can be a symptom of a number of different conditions of the head and neck. The brain tissue itself is not sensitive to pain because it lacks pain receptors. Rather, the pain is caused by disturbance of the...
, experienced by about 18% of drug arm participants compared to 11% of placebo participants. Headache was the only reported side effect to occur at a frequency greater than 5 percentage points above placebo. Other reported side effects and their rates for lorcaserin and placebo patients, respectively, were as follows: upper respiratory tract infection
Upper respiratory tract infection
Upper respiratory tract infections are the illnesses caused by an acute infection which involves the upper respiratory tract: nose, sinuses, pharynx or larynx...
(14.8% vs. 11.9%), nasopharyngitis (13.4% vs. 12.0%), sinusitis
Sinusitis
Sinusitis is inflammation of the paranasal sinuses, which may be due to infection, allergy, or autoimmune issues. Most cases are due to a viral infection and resolve over the course of 10 days...
(7.2% vs. 8.2%) and nausea
Nausea
Nausea , is a sensation of unease and discomfort in the upper stomach with an involuntary urge to vomit. It often, but not always, precedes vomiting...
(7.5% vs. 5.4%). Adverse events of depression
Depression (mood)
Depression is a state of low mood and aversion to activity that can affect a person's thoughts, behaviour, feelings and physical well-being. Depressed people may feel sad, anxious, empty, hopeless, helpless, worthless, guilty, irritable, or restless...
, anxiety
Anxiety
Anxiety is a psychological and physiological state characterized by somatic, emotional, cognitive, and behavioral components. The root meaning of the word anxiety is 'to vex or trouble'; in either presence or absence of psychological stress, anxiety can create feelings of fear, worry, uneasiness,...
and suicidal ideation
Suicidal ideation
Suicidal ideation is a common medical term for thoughts about suicide, which may be as detailed as a formulated plan, without the suicidal act itself. Although most people who undergo suicidal ideation do not commit suicide, some go on to make suicide attempts...
were infrequent and were reported at a similar rate in each treatment group.
On 15 September 2010 it was reported by national news-media that lorcaserin was associated with the development of cancer in laboratory rats.
Echocardiograms for valvulopathy
Regarding the risk of cardiac fibrosisCardiac fibrosis
Cardiac fibrosis refers to an abnormal thickening of the heart valves due to inappropriate proliferation of cardiac fibroblasts.Fibrocyte cells normally secrete collagen, and function to provide structural support for the heart...
, Arena Pharmaceuticals sought to rule out an increase in the rate of valvulopathy
Valvular heart disease
Valvular heart disease is any disease process involving one or more of the valves of the heart . Valve problems may be congenital or acquired...
of 20% or more because a number of anorectic drugs have been withdrawn for cardiovascular side-effects. In agreement with the FDA, Arena conducted regular and multiple echocardiograms of the phase III participants. At the 3, 6, and 12-month interval, the echocardiograms of participants of the BLOOM trial did not show any significant increase in valvulopathy over baseline, so the independent Echocardiographic Data Safety Monitoring Board (EDSMB) hired to monitor the trial allowed the trial to continue to the end. BLOOM participants received 18- and 24-month follow-up echocardiograms, but these results will not be reviewed by an EDSMB. The two other Phase 3 trials provide multiple and regular echocardiograms but they were not be reviewed by an EDSMB. BLOOM participants were pre-screened to exclude valvulopathy, but BLOSSOM and BLOOM-DM participants were not. Like BLOOM, BLOSSOM showed no significant increase in valvulopathy.
Rates of new FDA-defined valvulopathy in BLOOM were as follows: lorcaserin 10 mg twice daily (2.7%) and placebo (2.3%) at Week 52 and lorcaserin 10 mg twice daily (2.6%) and placebo (2.7%) at Week 104. For BLOSSOM, rates of new FDA-defined valvulopathy in BLOSSOM at Week 52 were as follows: lorcaserin 10 mg twice daily (2.0%), 10 mg once daily (1.4%) and placebo (2.0%).
Chemical properties
Lorcaserin is a benzazepineBenzazepine
Benzazepines are chemical compounds containing both a benzene and an azepine group.Examples include fenoldopam....
with a structure similar to dexfenfluramine
Dexfenfluramine
Dexfenfluramine, marketed as dexfenfluramine hydrochloride under the name Redux, is a serotoninergic anorectic drug: it reduces appetite by increasing the amount of extracellular serotonin in the brain...
, an anorectic drug that was withdrawn because of cardiovascular side-effects.
Financial aspects
In 2010, Arena Pharmaceuticals attended multiple biotechnology and pharmaceutical conferences and hosted several conference calls to discuss the Phase III results released so far. Market reaction to these results has been confused, driving it to a high of over $7 in early 2009 to a low of $2.61 in mid 2010. While management has reiterated its desire to partner lorcaserin with a large pharmaceutical company, in 2010 it has twice raised money from private hands, raising a total of nearly $60 million. For the first time, management has discussed the possibility of a go-it-alone strategy. Piper JaffrayPiper Jaffray
Piper Jaffray & Co. , often shortened to just Piper Jaffray or PiperJaffray, is a U.S. middle-market investment banking firm based in Minneapolis, Minnesota, and sells financial advice, investment products, and transaction execution within targeted sectors of the financial services marketplace...
has forecast lorcaserin sales of $3 billion in 2015. Arena has a very close financial relationship a group of private funds under the Deerfield umbrella name (Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P., Deerfield Partners, L.P., Deerfield International Limited, Deerfield Special Situations Fund, L.P., and Deerfield Special Situations Fund International Limited) under which Deerfield has lent Arena $100 million, purchased 11 million shares, and holds 28 million warrants to purchase additional shares at prices ranging from $3.23 to $5.48. On 15 September 2010 shares of Arena fell nearly 40%, from $6.85 to $4.13, on news that the lorcaserin has been linked to formation of malignant tumors in rats. On 16 September, an FDA advisory panel voted 9 to 5 to recommend against approval of the drug based on concerns over both safety and efficacy, trading on Arena's stock was stopped on that date, but after hours, the stock price fell about 40 percent.

