
Localized molecular orbitals
Encyclopedia
Localized molecular orbitals are molecular orbital
s which are concentrated in a limited spatial region of a molecule, for example a specific bond or a lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation
.
Standard ab initio quantum chemistry methods
lead to delocalized orbitals which in general extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combination
s of the delocalized orbitals, given by an appropriate unitary transformation
.
In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The localized orbital corresponding to one O-H bond is the sum of these two delocalized orbitals, and the localized orbital for the other O-H bond is their difference. Similarly, molecular orbital calculations show two nonbonding valence-shell orbitals: a roughly sp2 hybrid orbital in the plane of the molecule and a pure p orbital perpendicular to this plane. The tetrahedral sp3 hybrids of valence bond theory
and the electron pairs of VSEPR theory
can be compared to the sum and the difference of these nonbonding orbitals.
must have a form which satisfies the Pauli exclusion principle
such as a Slater determinant
(or linear combination of Slater determinants), and it can be shown that if two electrons are exchanged, such a function is unchanged by any unitary transformation of the doubly occupied orbitals.
upon a set of canonical molecular orbitals (CMO). The transformation usually involves the optimization (either minimization or maximization) of the expectation value of a specific operator. The generic form of the localization potential is:
 ,
,
where is the localization operator and
is the localization operator and  is a molecular spatial orbital. Many methodologies have been developed during the past decades, differing in the form of
is a molecular spatial orbital. Many methodologies have been developed during the past decades, differing in the form of 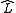 .
.
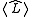 , where
, where  . This turns out to be equivalent to the easier task of maximizing
. This turns out to be equivalent to the easier task of maximizing  .
.
 , where
, where  .
.
Pipek-Mezey localization takes a slightly different approach, maximizing the sum of Mulliken charges:
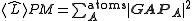 .
.
 bonds
bonds
and bonds
bonds
.
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s which are concentrated in a limited spatial region of a molecule, for example a specific bond or a lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation
Electronic correlation
Electronic correlation is the interaction between electrons in the electronic structure of a quantum system.- Atomic and molecular systems :...
.
Standard ab initio quantum chemistry methods
Ab initio quantum chemistry methods
Ab initio quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term ab initiowas first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene.The background is described by Parr...
lead to delocalized orbitals which in general extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combination
Linear combination
In mathematics, a linear combination is an expression constructed from a set of terms by multiplying each term by a constant and adding the results...
s of the delocalized orbitals, given by an appropriate unitary transformation
Unitary transformation
In mathematics, a unitary transformation may be informally defined as a transformation that respects the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation....
.
In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The localized orbital corresponding to one O-H bond is the sum of these two delocalized orbitals, and the localized orbital for the other O-H bond is their difference. Similarly, molecular orbital calculations show two nonbonding valence-shell orbitals: a roughly sp2 hybrid orbital in the plane of the molecule and a pure p orbital perpendicular to this plane. The tetrahedral sp3 hybrids of valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
and the electron pairs of VSEPR theory
VSEPR theory
Valence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
can be compared to the sum and the difference of these nonbonding orbitals.
Equivalence of localized and delocalized orbital descriptions
For molecules with a closed electron shell, in which each molecular orbital is doubly occupied, the localized and delocalized orbital descriptions are in fact equivalent and represent the same physical state. It might seem, again using the example of water, that placing two electrons in the first bond and two other electrons in the second bond is not the same as having four electrons free to move over both bonds. However in quantum mechanics all electrons are identical and cannot be distinguished as same or other. The total wavefunctionWavefunction
Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of...
must have a form which satisfies the Pauli exclusion principle
Pauli exclusion principle
The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
such as a Slater determinant
Slater determinant
In quantum mechanics, a Slater determinant is an expression that describes the wavefunction of a multi-fermionic system that satisfies anti-symmetry requirements and consequently the Pauli exclusion principle by changing sign upon exchange of fermions . It is named for its discoverer, John C...
(or linear combination of Slater determinants), and it can be shown that if two electrons are exchanged, such a function is unchanged by any unitary transformation of the doubly occupied orbitals.
Computation methods
Localized molecular orbitals (LMO) are obtained by unitary transformationUnitary transformation
In mathematics, a unitary transformation may be informally defined as a transformation that respects the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation....
upon a set of canonical molecular orbitals (CMO). The transformation usually involves the optimization (either minimization or maximization) of the expectation value of a specific operator. The generic form of the localization potential is:
 ,
,where
 is the localization operator and
is the localization operator and  is a molecular spatial orbital. Many methodologies have been developed during the past decades, differing in the form of
is a molecular spatial orbital. Many methodologies have been developed during the past decades, differing in the form of 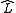 .
.Boys
Boys (also known as Foster-Boys) localization minimizes the spatial extent of the orbitals by minimizing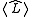 , where
, where  . This turns out to be equivalent to the easier task of maximizing
. This turns out to be equivalent to the easier task of maximizing  .
.Edminston-Ruedenberg
Edminston-Ruedenberg localization maximizes the electronic self-repulsion energy by maximizing , where
, where  .
.Pipek-Mezey
Pipek-Mezey localization takes a slightly different approach, maximizing the sum of Mulliken charges:
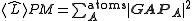 .
.Comparison
These three methods typically give very similar results, the main difference being that the Pipek-Mezey method does not mix bonds
bondsSigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
and
 bonds
bondsPi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
.

