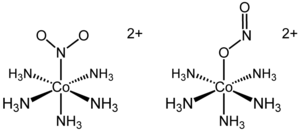
Linkage isomerism
Encyclopedia
Linkage isomerism is the existence of co-ordination compound
s that have the same composition differing with the connectivity of the metal to a ligand
.
Typical ligands that give rise to linkage isomers are:
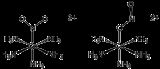
Examples of linkage isomers are violet-colored [(NH3)5Co-SCN]2+ and orange-colored [(NH3)5Co-NCS]2+. The isomerization of the S-bonded isomer to the N-bonded isomer occurs intramolecularly. In the complex, dichlorotetrakis(dimethyl sulfoxide)ruthenium(II), linkage isomerism of dimethyl sulfoxide ligands can be observed in the NMR spectrum due to the effect of S vs. O bonding on the methyl groups of DMSO. The proper notation for linkage isomerism is the kappa notation where the atom directly bonding to the metal is proceeded by the lowercase Greek letter kappa; κ. For example, NO2- is represented as nitrito-κ-N and nitrito-κ-O, replacing the old system of trivial names such as nitro and nitroso.
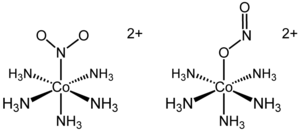
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
s that have the same composition differing with the connectivity of the metal to a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
.
Typical ligands that give rise to linkage isomers are:
- thiocyanateThiocyanateThiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
, SCN- - selenocyanate, SeCN-
- nitriteNitriteThe nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
, NO2- - sulfiteSulfiteSulfites are compounds that contain the sulfite ion SO. The sulfite ion is the conjugate base of bisulfite. Although the acid itself is elusive, its salts are widely used.-Structure:...
, SO32-
Examples of linkage isomers are violet-colored [(NH3)5Co-SCN]2+ and orange-colored [(NH3)5Co-NCS]2+. The isomerization of the S-bonded isomer to the N-bonded isomer occurs intramolecularly. In the complex, dichlorotetrakis(dimethyl sulfoxide)ruthenium(II), linkage isomerism of dimethyl sulfoxide ligands can be observed in the NMR spectrum due to the effect of S vs. O bonding on the methyl groups of DMSO. The proper notation for linkage isomerism is the kappa notation where the atom directly bonding to the metal is proceeded by the lowercase Greek letter kappa; κ. For example, NO2- is represented as nitrito-κ-N and nitrito-κ-O, replacing the old system of trivial names such as nitro and nitroso.
History
The first reported example of linkage isomerism had the formula [Co(NH3)5(NO2)]Cl2. The cationic cobalt complex exists in two separable linkage isomers. In the yellow-coloured isomer, the nitro ligand is bound through nitrogen. In the red linkage isomer, the nitrito is bound through one oxygen atom. The O-bonded isomer is often written as [Co(NH3)5(ONO)]2+. Although the existence of the isomers had been known since the late 1800s, only in 1907 was the structural difference explained. It was later shown that the red isomer converted to the yellow isomer upon UV-irradiation. In this particular example, the formation of the nitro isomer (Co-NO2) from the nitrito isomer (Co-ONO) occurs through the rearrangement of the molecular structure. Thus, no bonds are broken during isomerization.