
Limiting reagent
Encyclopedia
In a chemical reaction
, the limiting reagent, also known as the "limiting reactant", is the substance
which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess of the quantities required to react with the limiting reagent.
The limiting reagent must be identified in order to calculate the percentage yield
of a reaction, since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely.
Given the balanced chemical equation
which describes the reaction, there are several equivalent ways to identify the limiting reagent and evaluate the excess quantities of other reagents.
of benzene
, represented by the following chemical equation
:
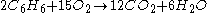
This means that 15 mol
molecular oxygen
(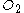 ) is required to react with 2 mol benzene (
) is required to react with 2 mol benzene (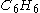 ).
).
The amount of oxygen required for other quantities of benzene can be calculated using cross-multiplication (the rule of three). For example,
if 1.5 mol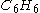 is present, 11.25 mol
is present, 11.25 mol 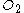 is required:
is required:
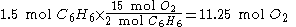
If in fact 18 mol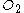 are present, there will be an excess of (18 - 11.25) = 6.75 mol of unreacted oxygen when all the benzene is consumed. Benzene is then the limiting reagent.
are present, there will be an excess of (18 - 11.25) = 6.75 mol of unreacted oxygen when all the benzene is consumed. Benzene is then the limiting reagent.
This conclusion can be verified by comparing the mole ratio of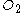 and
and 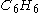 required by the balanced equation with the mole ratio actually present:
required by the balanced equation with the mole ratio actually present:
Since the actual ratio is larger than required,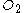 is the reagent in excess, which confirms that benzene is the limiting reagent.
is the reagent in excess, which confirms that benzene is the limiting reagent.
(Al) in the followng thermite reaction?
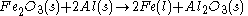
Since the reactant amounts are given in grams, they must be first converted into moles for comparison with the chemical equation, in order to determine how many moles of Fe can be produced from either reactant.
Moles produced of from reactant
from reactant 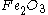
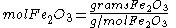
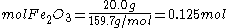
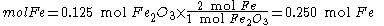
Moles produced of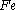 from reactant
from reactant 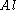
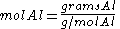
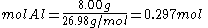
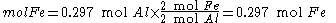
There is enough Al to produce 0.297 mol Fe, but only enough Fe2O3 to produce 0.250 mol Fe. This means that the amount of Fe actually produced is limited by the Fe2O3 present, which is therefore the limiting reagent.
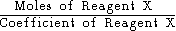
This suggests a shortcut which works for any number of reagents. Just calculate this formula for each reagent, and the reagent that has the lowest value of this formula is the limiting reagent.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, the limiting reagent, also known as the "limiting reactant", is the substance
Chemical substance
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess of the quantities required to react with the limiting reagent.
The limiting reagent must be identified in order to calculate the percentage yield
Yield (chemistry)
In chemistry, yield, also referred to as chemical yield and reaction yield, is the amount of product obtained in a chemical reaction. The absolute yield can be given as the weight in grams or in moles...
of a reaction, since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely.
Given the balanced chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
which describes the reaction, there are several equivalent ways to identify the limiting reagent and evaluate the excess quantities of other reagents.
Method 1: Comparison of reactant amounts
This method is most useful when there are only two reactants. One reactant (A) is chosen, and the balanced chemical equation is used to determine the amount of the other reactant (B) necessary to react with A. If the amount of B actually present exceeds the amount required, then B is in excess and A is the limiting reagent. If the amount of B present is less than required, then B is the limiting reagent.Example for two reactants
Consider the combustionCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, represented by the following chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
:
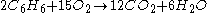
This means that 15 mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(
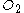 ) is required to react with 2 mol benzene (
) is required to react with 2 mol benzene (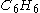 ).
).The amount of oxygen required for other quantities of benzene can be calculated using cross-multiplication (the rule of three). For example,
if 1.5 mol
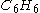 is present, 11.25 mol
is present, 11.25 mol 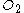 is required:
is required: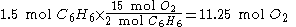
If in fact 18 mol
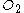 are present, there will be an excess of (18 - 11.25) = 6.75 mol of unreacted oxygen when all the benzene is consumed. Benzene is then the limiting reagent.
are present, there will be an excess of (18 - 11.25) = 6.75 mol of unreacted oxygen when all the benzene is consumed. Benzene is then the limiting reagent.This conclusion can be verified by comparing the mole ratio of
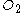 and
and 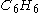 required by the balanced equation with the mole ratio actually present:
required by the balanced equation with the mole ratio actually present:- required:
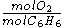 =
= 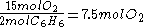
- actual:
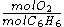 =
= 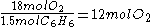
Since the actual ratio is larger than required,
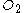 is the reagent in excess, which confirms that benzene is the limiting reagent.
is the reagent in excess, which confirms that benzene is the limiting reagent.Method 2: Comparison of product amounts which can be formed from each reactant
In this method the chemical equation is used to calculate the amount of one product which can be formed from each reactant in the amount present. This method can be extended to any number of reactants more easily than the first method.Example
Which reactant is limiting if 20.0 g of iron (III) oxide (Fe2O3) are reacted with 8.00 g aluminiumAluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
(Al) in the followng thermite reaction?
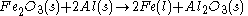
Since the reactant amounts are given in grams, they must be first converted into moles for comparison with the chemical equation, in order to determine how many moles of Fe can be produced from either reactant.
Moles produced of
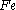 from reactant
from reactant 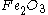
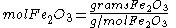
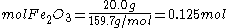
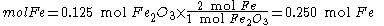
Moles produced of
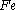 from reactant
from reactant 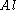
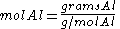
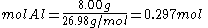
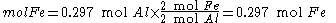
There is enough Al to produce 0.297 mol Fe, but only enough Fe2O3 to produce 0.250 mol Fe. This means that the amount of Fe actually produced is limited by the Fe2O3 present, which is therefore the limiting reagent.
Shortcut
It can be seen from the example above that the amount of product (Fe) formed from each reagent X (Fe2O3 or Al) is proportional to the quantity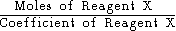
This suggests a shortcut which works for any number of reagents. Just calculate this formula for each reagent, and the reagent that has the lowest value of this formula is the limiting reagent.

