
Knorr quinoline synthesis
Encyclopedia
The Knorr quinoline synthesis is an intramolecular
organic reaction
converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid
. This reaction was first described by Ludwig Knorr
(1859 - 1921) in 1886
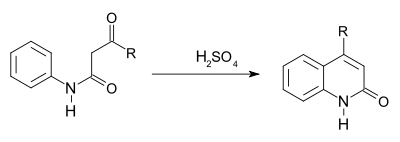 The reaction is a type of electrophilic aromatic substitution
The reaction is a type of electrophilic aromatic substitution
accompanied by elimination
of water. A 1964 study found that with certain reaction conditions formation of a 4-hydroxyquinoline is a competing reaction . For instance, the compound benzoylacetanilide (1) forms the 2-hydroxyquinoline (2) in a large excess of polyphosphoric acid (PPA) but 4-hydroxyquinoline 3 when the amount of PPA is small. A reaction mechanism
identified a N,O-dicationic intermediate A with excess acid capable of ring-closing and a monocationic intermediate B which fragments to aniline
and (ultimately to) acetophenone
. Aniline reacts with another equivalent of benzoylacetanilide before forming the 4-hydroxyquinoline.
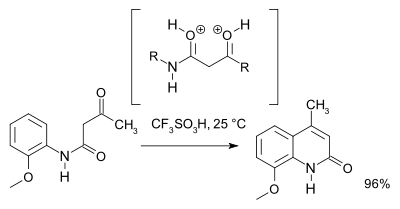
 A 2007 study revised the reaction mechanism and based on NMR spectroscopy
A 2007 study revised the reaction mechanism and based on NMR spectroscopy
and theoretical calculations favors a O,O-dicationic intermediate (a superelectrophile) over the N,O dicationic intermediate . For preparative purposes triflic acid is recommended:
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
converting a β-ketoanilide to a 2-hydroxyquinoline using sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. This reaction was first described by Ludwig Knorr
Ludwig Knorr
Ludwig Knorr was a German chemist. Together with Carl Paal, he discovered the Paal-Knorr synthesis, and the Knorr quinoline synthesis and Knorr pyrrole synthesis are also named after him. The synthesis in 1883 of the analgesic drug Antipyrin, now called Phenazone, was a commercial success...
(1859 - 1921) in 1886

Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
accompanied by elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of water. A 1964 study found that with certain reaction conditions formation of a 4-hydroxyquinoline is a competing reaction . For instance, the compound benzoylacetanilide (1) forms the 2-hydroxyquinoline (2) in a large excess of polyphosphoric acid (PPA) but 4-hydroxyquinoline 3 when the amount of PPA is small. A reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
identified a N,O-dicationic intermediate A with excess acid capable of ring-closing and a monocationic intermediate B which fragments to aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
and (ultimately to) acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
. Aniline reacts with another equivalent of benzoylacetanilide before forming the 4-hydroxyquinoline.

NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
and theoretical calculations favors a O,O-dicationic intermediate (a superelectrophile) over the N,O dicationic intermediate . For preparative purposes triflic acid is recommended:


