Isolobal principle
Encyclopedia
The isolobal principle is a strategy used in organometallic chemistry
to relate the structure of organic
and inorganic
molecular fragments in order to predict bond
ing properties of organometallic compounds. Roald Hoffmann
described molecular fragments as isolobal "if the number, symmetry
properties, approximate energy and shape of the frontier orbitals and the number of electron
s in them are similar – not identical, but similar." One can predict the bonding and reactivity of a lesser-known species from that of a better-known species if the two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds are analogues to isoelectronic compounds that share the same number of valence electron
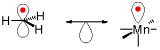
s and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
For his work on the isolobal analogy, Hoffmann was awarded the Nobel Prize in Chemistry
in 1981, which he shared with Kenichi Fukui
. In his Nobel Prize lecture, Hoffmann stressed that the isolobal analogy is a useful, yet simple, model and thus is bound to fail in certain instances.
s should satisfy the octet rule
when all bonding and nonbonding molecular orbitals (MOs) are filled and all antibonding MOs are empty. For example methane is a simple molecule from which to form a main group fragment. The removal of a hydrogen atom from methane generates a methyl radical. The molecule retains its molecular geometry
as the frontier orbital points in the direction of the missing hydrogen atom. Further removal of hydrogen results in the formation of a second frontier orbital. This process can be repeated until only one bond remains to the molecule's central atom. Figure 2 demonstrates this example of step-by-step generation of isolobal fragments.
The isolobal fragments of octahedral complexes, such as ML6, can be created in a similar fashion. Transition metal complexes should initially satisfy the eighteen electron rule, have no net charge, and their ligands should be two electron donors (Lewis bases). Consequently, the metal center for the ML6 starting point must be d6. Removal of a ligand is analogous to the removal of hydrogen of methane in the previous example resulting in a frontier orbital, which points toward the removed ligand. Cleaving the bond between the metal center and one ligand results in a ML5− radical complex. In order to satisfy the zero charge criteria the metal center must be changed. For example, a MoL6 complex is d6 and neutral. However, removing a ligand to form the first frontier orbital would result in a MoL5− complex because Mo has obtained an additional electron making it d7. To remedy this, Mo can be exchanged for Mn, which would from a neutral d7 complex in this case, as shown in Figure 3. This trend can continue until only one ligand is left coordinated to the metal center.
For example, consider Figure 5, which shows the production of frontier orbitals in tetrahedral and octahedral molecules.
As seen above, when a fragment is formed from CH4, one of the sp3 hybrid orbitals involved in bonding becomes a nonbonding singly occupied frontier orbital. The frontier orbital’s increased energy level is also shown in the figure. Similarly when starting with a metal complex such as d6–ML6, the d2sp3 hybrid orbitals are affected. Furthermore the t2g nonbonding metal orbitals are unaltered.
s, halogens or carbonyl
s. However, other types of ligands can be employed. If ligands donate multiple pairs of electrons, they will occupy multiple coordination sites. For example, the cyclopentadienyl
anion is a six-electron donor, so it occupies three coordination sites. Polydentate ligands can also be used in the analogy, such as ethylenediamine, a bidentate ligand, or triethylenetetramine, a tetradentate ligand.
In a similar sense, the addition or removal of electrons from two isolobal fragments results in two new isolobal fragments. Since Re(CO)5 is isolobal with CH3, [Re(CO)5]+ is isolobal with CH3+.
The analogy applies to other shapes besides tetrahedral and octahedral geometries. The derivations used in octahedral geometry are valid for most other geometries. The exception is square-planar because square-planar complexes typically abide by the 16-electron rule. Assuming ligands act as two-electron donors the metal center in square-planar molecules is d8. To relate an octahedral fragment, MLn, where M has a dx electron configuration to a square planar analogous fragment, the formula MLn−2 where M has a dx+2 electron configuration should be followed.
Further examples of the isolobal analogy in various shapes and forms are shown in Figure 8.
. Fe(CO)3 is isolobal with CH+. Therefore one can predict that CH+ will coordinate with cyclobutadiene in a similar fashion that Fe(CO)3 will. Thus the molecule C5H5+ can be envisioned regardless of its actuality.
Predicting the reactivity of complexes can also be accomplished using the isolobal analogy. From the simple expectation of two CH3 radicals reacting to form ethane one can use the analogy to predict M–C or M–M bonding such as (CH3)M(CO)5 and M2(CO)10, where M is d7.
Another application of the isolobal analogy is assisting in predicting reaction mechanism
s. As in the other applications the mechanisms of well-known reactions can be used to help predict mechanistic pathways of lesser-known reactions. There is no limit on the potential comparisons between organic and inorganic complexes. The analogy can flow in either direction (Organic to Inorganic) or within each division (Organic to Organic).
Arteaga-Müller et al. utilize the isolobal analogy to relate imido half-sandwich complexes with isoelectronic dicyclopentadienyl complexes. The isolobal relationship of the imido and the cyclopentadienyl ligands is the key to this comparison. The study found the reactivity of these two types of complexes to be similar although their catalytic abilities differed in some respects. This study shows that the isolobal analogy does not make perfect predictions between two isolobal fragments, as Hoffman warned in his Nobel Lecture.
Wu et al. apply the isolobal analogy to explore relationships involving structures, energies and magnetic properties between polyhedral boron carbonyls and their hydrocarbon relatives. As determined in this study, although isolobal, these two sets of molecules have significant differences in their strain energy
.
Goldman and Tyler used the isolobal analogy to determine the most likely mechanism for a deletion reaction. One of the products of the irradiation
of Cp
W(CO)3Me in the presence of PPh3 is CpW(CO)3−. The mechanism of said reaction was studied and theorized to be isolobal to the disproportionation
of metal-metal bonded dimers involving 19-valence electron intermediates. The reactions are composed of isolobal fragments and the key intermediates of both reactions are isolobal. Thus, the reaction pathways are mechanistically isolobal.
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
to relate the structure of organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
and inorganic
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
molecular fragments in order to predict bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
ing properties of organometallic compounds. Roald Hoffmann
Roald Hoffmann
Roald Hoffmann is an American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He currently teaches at Cornell University in Ithaca, New York.-Escape from the Holocaust:...
described molecular fragments as isolobal "if the number, symmetry
Symmetry
Symmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
properties, approximate energy and shape of the frontier orbitals and the number of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in them are similar – not identical, but similar." One can predict the bonding and reactivity of a lesser-known species from that of a better-known species if the two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds are analogues to isoelectronic compounds that share the same number of valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
For his work on the isolobal analogy, Hoffmann was awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1981, which he shared with Kenichi Fukui
Kenichi Fukui
Kenichi Fukui was a Japanese chemist.Kenichi Fukui was co-recipient of the Nobel Prize in Chemistry in 1981 with Roald Hoffmann, for their independent investigations into the mechanisms of chemical reactions...
. In his Nobel Prize lecture, Hoffmann stressed that the isolobal analogy is a useful, yet simple, model and thus is bound to fail in certain instances.
Construction of isolobal fragments
To begin to generate an isolobal fragment, the molecule needs to follow certain criteria. Molecules based around main group elementMain group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
s should satisfy the octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
when all bonding and nonbonding molecular orbitals (MOs) are filled and all antibonding MOs are empty. For example methane is a simple molecule from which to form a main group fragment. The removal of a hydrogen atom from methane generates a methyl radical. The molecule retains its molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
as the frontier orbital points in the direction of the missing hydrogen atom. Further removal of hydrogen results in the formation of a second frontier orbital. This process can be repeated until only one bond remains to the molecule's central atom. Figure 2 demonstrates this example of step-by-step generation of isolobal fragments.
The isolobal fragments of octahedral complexes, such as ML6, can be created in a similar fashion. Transition metal complexes should initially satisfy the eighteen electron rule, have no net charge, and their ligands should be two electron donors (Lewis bases). Consequently, the metal center for the ML6 starting point must be d6. Removal of a ligand is analogous to the removal of hydrogen of methane in the previous example resulting in a frontier orbital, which points toward the removed ligand. Cleaving the bond between the metal center and one ligand results in a ML5− radical complex. In order to satisfy the zero charge criteria the metal center must be changed. For example, a MoL6 complex is d6 and neutral. However, removing a ligand to form the first frontier orbital would result in a MoL5− complex because Mo has obtained an additional electron making it d7. To remedy this, Mo can be exchanged for Mn, which would from a neutral d7 complex in this case, as shown in Figure 3. This trend can continue until only one ligand is left coordinated to the metal center.
Relationship between tetrahedral and octahedral fragments
Isolobal fragments of tetrahedral and octahedral molecules can be related. Structures with the same number of frontier orbitals are isolobal to one another. For example, the methane with two hydrogen atoms removed, CH2 is isolobal to a d8 ML4 complex formed from an octahedral starting complex (Figure 4). Similar analogies can be made between either CH3 and d7–ML5 or CH and d9–ML3.MO theory dependence
Any sort of saturated molecule can be the starting point for generating isolobal fragments. The molecules' bonding and nonbonding MOs should be filled and the antibonding MOs empty. With each consecutive generation of a isolobal fragment, electrons are removed from the bonding orbitals and a frontier orbital is created. The frontier orbitals are at a higher energy level than the bonding and nonbonding MOs. Each frontier orbital contains one electron.For example, consider Figure 5, which shows the production of frontier orbitals in tetrahedral and octahedral molecules.
As seen above, when a fragment is formed from CH4, one of the sp3 hybrid orbitals involved in bonding becomes a nonbonding singly occupied frontier orbital. The frontier orbital’s increased energy level is also shown in the figure. Similarly when starting with a metal complex such as d6–ML6, the d2sp3 hybrid orbitals are affected. Furthermore the t2g nonbonding metal orbitals are unaltered.
Extensions of the analogy
The isolobal analogy has applications beyond simple octahedral complexes. It can be used with a variety of ligands, charged species and non-octahedral complexes.Ligands
Typical ligands used in the isolobal analogy are two-electron donors such as phosphinePhosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
s, halogens or carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s. However, other types of ligands can be employed. If ligands donate multiple pairs of electrons, they will occupy multiple coordination sites. For example, the cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
anion is a six-electron donor, so it occupies three coordination sites. Polydentate ligands can also be used in the analogy, such as ethylenediamine, a bidentate ligand, or triethylenetetramine, a tetradentate ligand.
Isoelectronic fragments
The isolobal analogy can also be used with isoelectronic fragments having the same coordination number, which allows charged species to be considered. For example, Re(CO)5 is isolobal with CH3 and therefore, [Ru(CO)5]+ and [Mo(CO)5]− are also isolobal with CH3. Any 17-electron metal complex would be isolobal in this example.In a similar sense, the addition or removal of electrons from two isolobal fragments results in two new isolobal fragments. Since Re(CO)5 is isolobal with CH3, [Re(CO)5]+ is isolobal with CH3+.
Non-octahedral complexes
| Octahedral MLn |
Square-planar MLn-2 |
|---|---|
| d6: Mo(CO)5 | d8: [PdCl3]- |
| d8: Os(CO)4 | d10: Ni(PR3)2 |
The analogy applies to other shapes besides tetrahedral and octahedral geometries. The derivations used in octahedral geometry are valid for most other geometries. The exception is square-planar because square-planar complexes typically abide by the 16-electron rule. Assuming ligands act as two-electron donors the metal center in square-planar molecules is d8. To relate an octahedral fragment, MLn, where M has a dx electron configuration to a square planar analogous fragment, the formula MLn−2 where M has a dx+2 electron configuration should be followed.
Further examples of the isolobal analogy in various shapes and forms are shown in Figure 8.
Applications and examples
Uses of the isolobal analogy include providing a short-cut to understanding electronic structure, predicting reactivity and reaction mechanisms, and a method of classifying molecules. Applications are typically utilized to make connections between well-known systems and less familiar systems. For example, the possibility of unsynthesized compounds can be imagined from those of known molecular conformations. The isolobal analogy does not guarantee these products are capable of being produced, but only proposes a possibility. Consider the molecule Fe(CO)3 complexed with cyclobutadieneCyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
. Fe(CO)3 is isolobal with CH+. Therefore one can predict that CH+ will coordinate with cyclobutadiene in a similar fashion that Fe(CO)3 will. Thus the molecule C5H5+ can be envisioned regardless of its actuality.
Predicting the reactivity of complexes can also be accomplished using the isolobal analogy. From the simple expectation of two CH3 radicals reacting to form ethane one can use the analogy to predict M–C or M–M bonding such as (CH3)M(CO)5 and M2(CO)10, where M is d7.
Another application of the isolobal analogy is assisting in predicting reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s. As in the other applications the mechanisms of well-known reactions can be used to help predict mechanistic pathways of lesser-known reactions. There is no limit on the potential comparisons between organic and inorganic complexes. The analogy can flow in either direction (Organic to Inorganic) or within each division (Organic to Organic).
Arteaga-Müller et al. utilize the isolobal analogy to relate imido half-sandwich complexes with isoelectronic dicyclopentadienyl complexes. The isolobal relationship of the imido and the cyclopentadienyl ligands is the key to this comparison. The study found the reactivity of these two types of complexes to be similar although their catalytic abilities differed in some respects. This study shows that the isolobal analogy does not make perfect predictions between two isolobal fragments, as Hoffman warned in his Nobel Lecture.
Wu et al. apply the isolobal analogy to explore relationships involving structures, energies and magnetic properties between polyhedral boron carbonyls and their hydrocarbon relatives. As determined in this study, although isolobal, these two sets of molecules have significant differences in their strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
.
Goldman and Tyler used the isolobal analogy to determine the most likely mechanism for a deletion reaction. One of the products of the irradiation
Irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to...
of Cp
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
W(CO)3Me in the presence of PPh3 is CpW(CO)3−. The mechanism of said reaction was studied and theorized to be isolobal to the disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
of metal-metal bonded dimers involving 19-valence electron intermediates. The reactions are composed of isolobal fragments and the key intermediates of both reactions are isolobal. Thus, the reaction pathways are mechanistically isolobal.

