Iron-sulfur protein
Encyclopedia
Iron-sulfur proteins are protein
s characterized by the presence of iron-sulfur clusters containing sulfide
-linked di-, tri-, and tetrairon centers in variable oxidation state
s. Iron-sulfur clusters are found in a variety of metalloprotein
s, such as the ferredoxin
s, as well as NADH dehydrogenase
, hydrogenase
s, Coenzyme Q - cytochrome c reductase
, Succinate - coenzyme Q reductase
and nitrogenase
. Iron-sulfur clusters are best known for their role in the oxidation-reduction reactions of mitochondrial electron transport. Both Complex I and Complex II of oxidative phosphorylation
have multiple Fe-S clusters. They have many other functions including catalysis as illustrated by aconitase
, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid
and biotin
. Additionally some Fe-S proteins regulate gene expression. Fe-S proteins are vulnerable to attack by biogenic nitric oxide
.
The prevalence of these proteins on the metabolic pathways of most organisms lead some scientists to theorize that iron-sulfur compounds had a significant role in the origin of life in the Iron-sulfur world theory
.
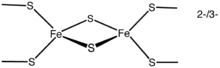 The simplest polymetallic system, the [Fe2S2] cluster, is constituted by two iron ions bridged by two sulfide ions and coordinated by four cysteinyl ligand
The simplest polymetallic system, the [Fe2S2] cluster, is constituted by two iron ions bridged by two sulfide ions and coordinated by four cysteinyl ligand
s (in Fe2S2 ferredoxin
s) or by two cysteine
s and two histidine
s (in Rieske protein
s). The oxidized proteins contain two Fe3+ ions, whereas the reduced proteins contain one Fe3+ and one Fe2+ ion. These species exist in two oxidation states, (FeIII)2 and FeIIIFeII.
-type structure. The Fe centers are typically further coordinated by cysteinyl ligands. The [Fe4S4] electron-transfer proteins ([Fe4S4] ferredoxin
s) may be further subdivided into low-potential (bacterial-type) and high-potential (HiPIP) ferredoxins. Low- and high-potential ferredoxins are related by the following redox scheme:
In HiPIP, the cluster shuttles between [2Fe3+, 2Fe2+] (Fe4S42+) and [3Fe3+, Fe2+] (Fe4S43+). The potentials for this redox couple range from 0.4 to 0.1 V. In the bacterial ferredoxins, the pair of oxidation states are [Fe3+, 3Fe2+] (Fe4S4+) and [2Fe3+, 2Fe2+] (Fe4S42+). The potentials for this redox couple range from -0.3 to -0.7 V. The two families of 4Fe-4S clusters share the Fe4S42+ oxidation state. The difference in the redox couples is attributed to the degree of hydrogen bonding, which strongly modifies the basicity of the cysteinyl thiolate ligands. A further redox couple, which is still more reducing than the bacterial ferredoxins is implicated in the nitrogenase
.
Some 4Fe-4S clusters bind substrates and are thus classified as enzymes. In aconitase
, the Fe-S cluster binds aconitate at the one Fe centre that lacks a thiolate ligand. The cluster does not undergo redox, but serves as a Lewis acid
catalyst to convert aconitate to isocitrate. In the radical-SAM enzymes, the cluster binds and reduces S-adenosylmethionine to generate a radical, which is involved in many biosyntheses.
possesses an [Fe3S4] and is activated by addition of Fe2+ and reductant.
. Carbon monooxide dehydrogenase and the [FeFe]-hydrogenase
also feature unusual Fe-S clusters.
The biogenesis of iron sulfur clusters has been studied most extensively in the bacteria E. coli
and A. vinelandii
and yeast S. cerevisiae
. At least three different biosynthetic systems have been identified so far, namely nif, suf, and isc systems, which were first identified in bacteria. The nif system is responsible for the clusters in the enzyme nitrogenase. The suf and isc systems are more general with the isc-related proteins being present only in the animal kingdom. The yeast isc system is the best described. Several proteins constitute the biosynthetic machinery via the isc pathway. The process occurs in two major steps:
(1) the Fe/S cluster is assembled on a scaffold protein followed by (2) transfer of the preformed cluster to the recipient proteins.
The first step of this process occurs in the cytoplasm
of prokaryotic
organisms or in the mitochondria of eukaryotic
organisms. In the higher organisms the clusters are therefore transported out of the mitochondrion to be incorporated into the extramitochondrial enzymes. These organisms also possess a set of proteins involved in the Fe/S clusters transport and incorporation processes that are not homologous to proteins found in prokaryotic systems.
and coworkers. Treatment of iron salts with a mixture of thiolates and sulfide affords derivatives such as (Et4N
)2Fe4S4(SCH2Ph)4].
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s characterized by the presence of iron-sulfur clusters containing sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
-linked di-, tri-, and tetrairon centers in variable oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s. Iron-sulfur clusters are found in a variety of metalloprotein
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
s, such as the ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
s, as well as NADH dehydrogenase
NADH dehydrogenase
NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q...
, hydrogenase
Hydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
s, Coenzyme Q - cytochrome c reductase
Coenzyme Q - cytochrome c reductase
In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are dihydroquinone and ferri- cytochrome c, whereas its 3 products are quinone , ferro- cytochrome c, and H+.This enzyme belongs to the family of...
, Succinate - coenzyme Q reductase
Succinate - coenzyme Q reductase
Succinate dehydrogenase or succinate-coenzyme Q reductase or Complex II is an enzyme complex, bound to the inner mitochondrial membrane of mammalian mitochondria and many bacterial cells...
and nitrogenase
Nitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
. Iron-sulfur clusters are best known for their role in the oxidation-reduction reactions of mitochondrial electron transport. Both Complex I and Complex II of oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
have multiple Fe-S clusters. They have many other functions including catalysis as illustrated by aconitase
Aconitase
Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.- Function :...
, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid
Lipoic acid
Lipoic acid , also known as α-lipoic acid and Alpha Lipoic Acid is an organosulfur compound derived from octanoic acid. LA contains two vicinal sulfur atoms attached via a disulfide bond and is thus considered to be oxidized...
and biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
. Additionally some Fe-S proteins regulate gene expression. Fe-S proteins are vulnerable to attack by biogenic nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
.
The prevalence of these proteins on the metabolic pathways of most organisms lead some scientists to theorize that iron-sulfur compounds had a significant role in the origin of life in the Iron-sulfur world theory
Iron-sulfur world theory
The iron-sulfur world theory is a set of proposals for the origin of life and the early evolution of life advanced by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The theory...
.
Structural motifs
In almost all Fe-S proteins, the Fe centers are tetrahedral and the terminal ligands are thiolato sulfur centers from cysteinyl residues. The sulfide groups are either two- or three-coordinated. Three distinct kinds of Fe-S clusters with these features are most common.2Fe-2S clusters

Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s (in Fe2S2 ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
s) or by two cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
s and two histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
s (in Rieske protein
Rieske protein
Rieske proteins are iron-sulfur protein components of cytochrome bc1 complexes and cytochrome b6f complexes which were first discovered and isolated by John S. Rieske and co-workers in 1964. It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues...
s). The oxidized proteins contain two Fe3+ ions, whereas the reduced proteins contain one Fe3+ and one Fe2+ ion. These species exist in two oxidation states, (FeIII)2 and FeIIIFeII.
4Fe-4S clusters
A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubaneCubane
Cubane is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton, a...
-type structure. The Fe centers are typically further coordinated by cysteinyl ligands. The [Fe4S4] electron-transfer proteins ([Fe4S4] ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
s) may be further subdivided into low-potential (bacterial-type) and high-potential (HiPIP) ferredoxins. Low- and high-potential ferredoxins are related by the following redox scheme:
In HiPIP, the cluster shuttles between [2Fe3+, 2Fe2+] (Fe4S42+) and [3Fe3+, Fe2+] (Fe4S43+). The potentials for this redox couple range from 0.4 to 0.1 V. In the bacterial ferredoxins, the pair of oxidation states are [Fe3+, 3Fe2+] (Fe4S4+) and [2Fe3+, 2Fe2+] (Fe4S42+). The potentials for this redox couple range from -0.3 to -0.7 V. The two families of 4Fe-4S clusters share the Fe4S42+ oxidation state. The difference in the redox couples is attributed to the degree of hydrogen bonding, which strongly modifies the basicity of the cysteinyl thiolate ligands. A further redox couple, which is still more reducing than the bacterial ferredoxins is implicated in the nitrogenase
Nitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
.
Some 4Fe-4S clusters bind substrates and are thus classified as enzymes. In aconitase
Aconitase
Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.- Function :...
, the Fe-S cluster binds aconitate at the one Fe centre that lacks a thiolate ligand. The cluster does not undergo redox, but serves as a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst to convert aconitate to isocitrate. In the radical-SAM enzymes, the cluster binds and reduces S-adenosylmethionine to generate a radical, which is involved in many biosyntheses.
3Fe-4S clusters
Proteins are also known to contain [Fe3S4] centres, which feature one iron less than the more common [Fe4S4] cores. Three sulfide ions bridge two iron ions each, while the fourth sulfide bridges three iron ions. Their formal oxidation states may vary from [Fe3S4]+ (all-Fe3+ form) to [Fe3S4]2- (all-Fe2+ form). In a number of iron-sulfur proteins, the [Fe4S4] cluster can be reversibly converted by oxidation and loss of one iron ion to a [Fe3S4] cluster. E.g., the inactive form of aconitaseAconitase
Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.- Function :...
possesses an [Fe3S4] and is activated by addition of Fe2+ and reductant.
Other Fe-S clusters
More complex polymetallic systems are common. Examples include both the 8Fe and the 7Fe clusters in nitrogenaseNitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
. Carbon monooxide dehydrogenase and the [FeFe]-hydrogenase
Hydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
also feature unusual Fe-S clusters.
Biosynthesis
The biosynthesis of the Fe-S clusters has been well studied.The biogenesis of iron sulfur clusters has been studied most extensively in the bacteria E. coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
and A. vinelandii
Azotobacter vinelandii
Azotobacter vinelandii is diazotroph that can fix nitrogen while grown aerobically. It is a genetically tractable system that is used to study nitrogen fixation...
and yeast S. cerevisiae
Saccharomyces cerevisiae
Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes...
. At least three different biosynthetic systems have been identified so far, namely nif, suf, and isc systems, which were first identified in bacteria. The nif system is responsible for the clusters in the enzyme nitrogenase. The suf and isc systems are more general with the isc-related proteins being present only in the animal kingdom. The yeast isc system is the best described. Several proteins constitute the biosynthetic machinery via the isc pathway. The process occurs in two major steps:
(1) the Fe/S cluster is assembled on a scaffold protein followed by (2) transfer of the preformed cluster to the recipient proteins.
The first step of this process occurs in the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
of prokaryotic
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
organisms or in the mitochondria of eukaryotic
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
organisms. In the higher organisms the clusters are therefore transported out of the mitochondrion to be incorporated into the extramitochondrial enzymes. These organisms also possess a set of proteins involved in the Fe/S clusters transport and incorporation processes that are not homologous to proteins found in prokaryotic systems.
Synthetic analogues
Synthetic analogues of the naturally occurring Fe-S clusters were first reported by HolmRichard H. Holm
Richard Hadley Holm , also known as R. H. Holm, is an American inorganic chemist.Professor Holm received his Ph.D. from the Massachusetts Institute of Technology in 1959 under the direction of F. Albert Cotton. After the completion of his degree, he joined the chemistry faculty at Harvard University...
and coworkers. Treatment of iron salts with a mixture of thiolates and sulfide affords derivatives such as (Et4N
Tetraethylammonium
Tetraethylammonium is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom. Like other members of its class, it can be used to alter a compound's solubility by displacing hard acids with this comparatively softer acid...
)2Fe4S4(SCH2Ph)4].

