
HB plot
Encyclopedia
Knowledge of the relationship between a protein
's structure and its dynamic behavior is essential for understanding protein function. The description of a protein three dimensional structure as a network of hydrogen bonding interactions (HB plot) was introduced as a tool for exploring protein structure and function. By analyzing the network of tertiary interactions the possible spread of information within a protein can be investigated.
HB plot offers a simple way of analyzing protein secondary structure
and tertiary structure
. Hydrogen bonds stabilizing secondary structural elements (secondary hydrogen bond
s) and those formed between distant amino acid
residues - defined as tertiary hydrogen bond
s - can be easily distinguished in HB plot, thus, amino acid residues involved in stabilizing protein structure
and function can be identified. By analyzing the network of tertiary interactions the possible spread of information within a protein can be investigated as well.
and side chain-side chain hydrogen bonding interactions. Bifurcated hydrogen bonds and multiple hydrogen bonds between amino acid
residues; and intra- and interchain hydrogen bonds are also indicated on the plots. Three classes of hydrogen bondings are distinguished by color coding; short (distance smaller than 2.5 Å
between donor and acceptor), intermediate (between 2.5 Å and 3.2 Å) and long hydrogen bonds (greater than 3.2 Å).
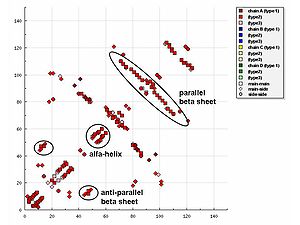 In representations of the HB plot, characteristic patterns of secondary structure
In representations of the HB plot, characteristic patterns of secondary structure
elements can be recognised easily, as follows:
-metabolizing membrane
-bound heme
-containing enzymes that use molecular oxygen
and electrons from NADPH cytochrome P450 reductase to oxidize their substrate
s. CYP2B4, a member of the cytochrome P450 family is the only protein within this family, whose X-ray structure
in both open 11 and closed form 12 is published. The comparison of the open and closed structures of CYP2B4 structures reveals large-scale conformational
rearrangement between the two states, with the greatest conformational change around the residues 215-225, which is widely open in ligand-free state and shut after ligand binding; and the region around loop C near the heme.
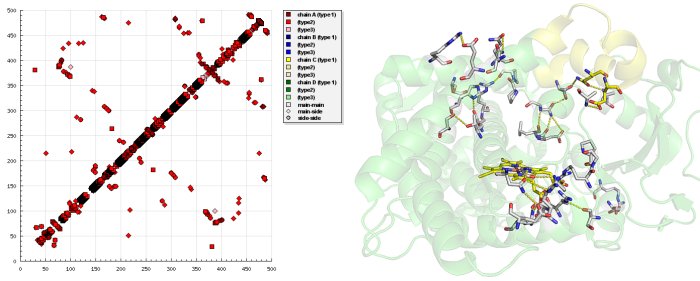 Examining the HB plot of the closed and open state of CYP2B4 revealed that the rearrangement of tertiary hydrogen bonds was in excellent agreement with the current knowledge of the cytochrome P450 catalytic cycle
Examining the HB plot of the closed and open state of CYP2B4 revealed that the rearrangement of tertiary hydrogen bonds was in excellent agreement with the current knowledge of the cytochrome P450 catalytic cycle
.
The first step in P450 catalytic cycle is identified as substrate binding. Preliminary binding of a ligand near to the entrance breaks hydrogen bonds S212-E474, S207-H172 in the open form of CYP2B4 and hydrogen bonds E218-A102, Q215-L51 are formed that fix the entrance in the closed form as the HB plot reveals.
The second step is the transfer of the first electron from NADPH via an electron transfer chain. For the electron transfer a conformational change occurs that triggers interaction of the P450 with the NADPH cytochrome P450 reductase. Breaking of hydrogen bonds between S128-N287, S128-T291, L124-N287 and forming S96-R434, A116-R434, R125-I435, D82-R400 at the NADPH cytochrome P450 reductase binding site
—as seen in HB plot—transform CYP2B4 to a conformation state, where binding of NADPH cytochrome P450 reductase occurs.
In the third step, oxygen enters CYP2B4 in the closed state - the state where newly formed hydrogen bonds S176-T300, H172-S304, N167-R308 open a tunnel which is exactly the size and shape of an oxygen
molecule.
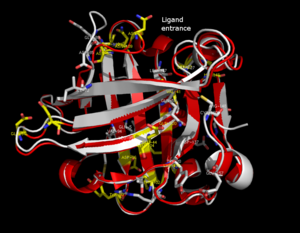 The lipocalin
The lipocalin
family is a large and diverse family of proteins with functions as small hydrophobic molecule transporters. Beta-lactoglobulin
is a typical member of the lipocalin family. Beta-lactoglobulin was found to have a role in the transport of hydrophobic ligands such as retinol
or fatty acid
s. Its crystal structure
were determined [e.g. Qin, 1998] with different ligands and in ligand-free form as well. The crystal structures determined so far reveal that the typical lipocalin contains eight-stranded antiparallel
-barrel arranged to form a conical central cavity in which the hydrophobic ligand is bound. The structure of beta-lactoglobulin reveals that the barrel-form structure with the central cavity of the protein has an "entrance" surrounded by five beta-loops with centers around 26, 35, 63, 87, and 111, which undergo a conformational change during the ligand binding and close the cavity.
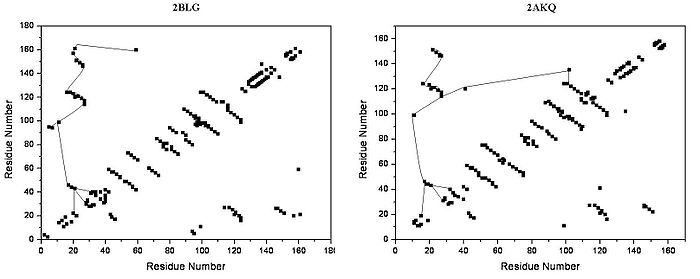
The overall shape of beta-lactoglobulin is characteristic of the lipocalin family. In the absence of alpha-helices, the main diagonal almost disappears and the cross-diagonals representing the beta-sheets dominate the plot. Relatively low number of tertiary hydrogen bonds can be found in the plot, with three high-density regions, one of which is connected to a loop at the residues around 63, a second is connected to the loop around 87, and a third region which is connected to the regions 26 and 35. The fifth loop around 111 is represented only one tertiary hydrogen bond in the HB plot.
In the three-dimensional structure, tertiary hydrogen bonds are formed (1) near to the entrance, directly involved in conformational rearrangement during ligand binding; and (2) at the bottom of the "barrel".
HB plots of the open and closed forms of beta-lactoglobulin are very similar, all unique motifs can be recognized in both forms. Difference in HB plots of open and ligand-bound form show few important individual changes in tertiary hydrogen bonding pattern. Especially, the formation of hydrogen bonds between Y20-E157 and S21-H161 in closed form might be crucial in conformational rearrangement. These hydrogen bonds lie at the bottom of the cavity, which suggests that the closure of the entrance of a lipocalin starts when a ligand reached the bottom of the cavity and broke hydrogen bonds R123-Y99, R123-T18, and V41-Q120. Lipocalins are known to have very low sequence similarity with high structural similarity. The only conserved regions are exactly the region around 20 and 160 with an unknown role.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
's structure and its dynamic behavior is essential for understanding protein function. The description of a protein three dimensional structure as a network of hydrogen bonding interactions (HB plot) was introduced as a tool for exploring protein structure and function. By analyzing the network of tertiary interactions the possible spread of information within a protein can be investigated.
HB plot offers a simple way of analyzing protein secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
and tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
. Hydrogen bonds stabilizing secondary structural elements (secondary hydrogen bond
Secondary hydrogen bond
Hydrogen bonding interaction in proteins plays a crucial role in protein folding, dynamics and function. The locally ordered structure, the so-called secondary structure of a protein is created by hydrogen bonding within the protein backbone...
s) and those formed between distant amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues - defined as tertiary hydrogen bond
Tertiary hydrogen bond
Hydrogen bonding interaction in proteins plays a crucial role in protein folding, dynamics and function. A few percent of hydrogen bonds connect distant amino acid residues and are not involved in secondary structure stabilization. These hydrogen bonds have special role in the proteins and called...
s - can be easily distinguished in HB plot, thus, amino acid residues involved in stabilizing protein structure
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
and function can be identified. By analyzing the network of tertiary interactions the possible spread of information within a protein can be investigated as well.
Features
The plot distinguishes between main chain-main chain, main chain-side chainSide chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
and side chain-side chain hydrogen bonding interactions. Bifurcated hydrogen bonds and multiple hydrogen bonds between amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues; and intra- and interchain hydrogen bonds are also indicated on the plots. Three classes of hydrogen bondings are distinguished by color coding; short (distance smaller than 2.5 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
between donor and acceptor), intermediate (between 2.5 Å and 3.2 Å) and long hydrogen bonds (greater than 3.2 Å).
Secondary structure elements in HB plot

Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
elements can be recognised easily, as follows:
- Helices can be identified as strips directly adjacent to the diagonal.
- Antiparallel beta sheets appear in HB plot as cross-diagonal.
- Parallel beta sheets appears in the HB plot as parallel to the diagonal.
- LoopLoop (graph theory)In graph theory, a loop is an edge that connects a vertex to itself. A simple graph contains no loops....
s appear as breaks in the diagonal between the cross-diagonal beta-sheet motifs.
Cytochrome P450s
The cytochrome P450s (P450s) are xenobioticXenobiotic
A xenobiotic is a chemical which is found in an organism but which is not normally produced or expected to be present in it. It can also cover substances which are present in much higher concentrations than are usual...
-metabolizing membrane
Membrane (selective barrier)
A membrane is a layer of material which serves as a selective barrier between two phases and remains impermeable to specific particles, molecules, or substances when exposed to the action of a driving force...
-bound heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
-containing enzymes that use molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and electrons from NADPH cytochrome P450 reductase to oxidize their substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
s. CYP2B4, a member of the cytochrome P450 family is the only protein within this family, whose X-ray structure
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
in both open 11 and closed form 12 is published. The comparison of the open and closed structures of CYP2B4 structures reveals large-scale conformational
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
rearrangement between the two states, with the greatest conformational change around the residues 215-225, which is widely open in ligand-free state and shut after ligand binding; and the region around loop C near the heme.

Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
.
The first step in P450 catalytic cycle is identified as substrate binding. Preliminary binding of a ligand near to the entrance breaks hydrogen bonds S212-E474, S207-H172 in the open form of CYP2B4 and hydrogen bonds E218-A102, Q215-L51 are formed that fix the entrance in the closed form as the HB plot reveals.
The second step is the transfer of the first electron from NADPH via an electron transfer chain. For the electron transfer a conformational change occurs that triggers interaction of the P450 with the NADPH cytochrome P450 reductase. Breaking of hydrogen bonds between S128-N287, S128-T291, L124-N287 and forming S96-R434, A116-R434, R125-I435, D82-R400 at the NADPH cytochrome P450 reductase binding site
Binding site
In biochemistry, a binding site is a region on a protein, DNA, or RNA to which specific other molecules and ions—in this context collectively called ligands—form a chemical bond...
—as seen in HB plot—transform CYP2B4 to a conformation state, where binding of NADPH cytochrome P450 reductase occurs.
In the third step, oxygen enters CYP2B4 in the closed state - the state where newly formed hydrogen bonds S176-T300, H172-S304, N167-R308 open a tunnel which is exactly the size and shape of an oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
molecule.
Lipocalin family

Lipocalin
The lipocalins are a family of proteins which transport small hydrophobic molecules such as steroids, bilins, retinoids, and lipids. They share limited regions of sequence homology and a common tertiary structure architecture...
family is a large and diverse family of proteins with functions as small hydrophobic molecule transporters. Beta-lactoglobulin
Beta-lactoglobulin
right|thumbnail|300px|Structure of a β-lactoglobulin subunit Ribbons denote the [[secondary structure]], with arrows for beta strands and spirals for alpha-helices. Rendered with [[Kinemage]]...
is a typical member of the lipocalin family. Beta-lactoglobulin was found to have a role in the transport of hydrophobic ligands such as retinol
Retinol
Retinol is one of the animal forms of vitamin A. It is a diterpenoid and an alcohol. It is convertible to other forms of vitamin A, and the retinyl ester derivative of the alcohol serves as the storage form of the vitamin in animals....
or fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s. Its crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
were determined [e.g. Qin, 1998] with different ligands and in ligand-free form as well. The crystal structures determined so far reveal that the typical lipocalin contains eight-stranded antiparallel
Antiparallel (biochemistry)
In biochemistry, two molecules are antiparallel if they run side-by-side in opposite directions or when both strands are complimentary to each other....
-barrel arranged to form a conical central cavity in which the hydrophobic ligand is bound. The structure of beta-lactoglobulin reveals that the barrel-form structure with the central cavity of the protein has an "entrance" surrounded by five beta-loops with centers around 26, 35, 63, 87, and 111, which undergo a conformational change during the ligand binding and close the cavity.
The overall shape of beta-lactoglobulin is characteristic of the lipocalin family. In the absence of alpha-helices, the main diagonal almost disappears and the cross-diagonals representing the beta-sheets dominate the plot. Relatively low number of tertiary hydrogen bonds can be found in the plot, with three high-density regions, one of which is connected to a loop at the residues around 63, a second is connected to the loop around 87, and a third region which is connected to the regions 26 and 35. The fifth loop around 111 is represented only one tertiary hydrogen bond in the HB plot.
In the three-dimensional structure, tertiary hydrogen bonds are formed (1) near to the entrance, directly involved in conformational rearrangement during ligand binding; and (2) at the bottom of the "barrel".
HB plots of the open and closed forms of beta-lactoglobulin are very similar, all unique motifs can be recognized in both forms. Difference in HB plots of open and ligand-bound form show few important individual changes in tertiary hydrogen bonding pattern. Especially, the formation of hydrogen bonds between Y20-E157 and S21-H161 in closed form might be crucial in conformational rearrangement. These hydrogen bonds lie at the bottom of the cavity, which suggests that the closure of the entrance of a lipocalin starts when a ligand reached the bottom of the cavity and broke hydrogen bonds R123-Y99, R123-T18, and V41-Q120. Lipocalins are known to have very low sequence similarity with high structural similarity. The only conserved regions are exactly the region around 20 and 160 with an unknown role.

External links
- HB Plot — a free online HB Plot program

