
Selenol
Encyclopedia

Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that contain the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
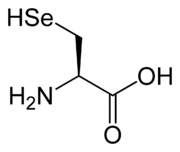
with the connectivity C-Se-H. Selenols are sometimes also called selenamercaptans, selenathiols, and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member is the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
selenocysteine
Selenocysteine
Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
.
Structure, bonding, properties
Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C-Se-H angle approaches 90° as it does in hydrogen selenideHydrogen selenide
Hydrogen selenide is the inorganic compound with the formula H2Se. It is the simplest and virtually the only hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit: 0.05 ppm over an 8 hour period...
(H2Se). The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se-C bond energy
Bond energy
In chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
is weaker than the S-H bond, consequently selenols are easily oxidized and serve as H-atom donors. Also reflecting the relative weakness of bonds to Se, selenols are about 1000x stronger acids than are thiols: the pKa of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affords the selenoate anion, RSe-, most examples of which are highly nucleophilic and rapidly oxidized by air.
The boiling points of selenols tend to be slightly greater than for thiols owing to the increased importance of van der Waals bonding, which is stronger for larger atoms. Volatile selenols have highly offensive odors.
Applications and occurrence
Selenols enjoy few commercial applications, being limited by the high toxicity of selenium as well as the sensitivity of the Se-H bond. Their conjugate bases, the selenoates, do enjoy limited applications in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Biochemical role
Selenols are important in certain biological processes. Three enzymes found in mammals contain selenols at their active sites: glutathione peroxidaseGlutathione peroxidase
Glutathione peroxidase is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage...
, iodothyronine deiodinase, and thioredoxin reductase
Thioredoxin reductase
Thioredoxin Reductases are the only known enzymes to reduce thioredoxin . Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Both classes are flavoproteins which function as homodimers...
. The selenols in these proteins are part of the essential amino acid
Essential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
selenocysteine
Selenocysteine
Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
.
Preparation
Selenols are prepared usually by the reaction of organolithium reagentOrganolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s or Grignard reagents with elemental Se. For example, benzeneselenol
Benzeneselenol
Benzeneselenol is the chemical compound with the formula C6H5SeH, often abbreviated PhSeH. This intensely malodorous liquid is a useful reagent in organic synthesis.-Synthesis and basic properties:...
is generated by the reaction of phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
with selenium followed by acidicifation:
Another preparative route to selenols involves the alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of selenourea
Selenourea
Selenourea is the organoselenium compound with the formula SeC2. It is a white solid. This compound features a rare example of a stable, unhindered carbon-selenium double bond. The compound is used in the synthesis of selenium heterocycles...
, followed by hydrolysis. Selenols are often generated by reduction of diselenides followed by protonation of the resulting selenoate:
- 2 RSeSeR + 2 LiHB(C2H5)3 → 2 RSeLi + 2 B(C2H5)3 + H2
- RSeLi + HCl → RSeH + LiCl
Reactions
Selenols are easily oxidized to diselenides, compounds containing an Se-Se bond. For example treatment of benzeneselenol with bromine gives diphenyl diselenideDiphenyl diselenide
Diphenyl diselenide is the chemical compound with the formula 2Se2, abbreviated Ph2Se2 This orange-coloured solid is the oxidized derivative of benzeneselenol...
.
- 2 C6H5SeH + Br2 → (C6H5Se)2 + 2 HBr

