
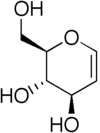
Glycal
Encyclopedia
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
derivatives of sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s having a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
between carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s 1 and 2 of the ring. The term “glycal” should not be used for an unsaturated
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
sugar that has a double bond in any position other than between carbon atoms 1 and 2.
History of Glycals
The first glycal was synthesizedChemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
by Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
and Karl Zach in 1913. They synthesized this 1,2-unsaturated sugar from D-glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and named their product D-glucal. Fischer believed he had synthesized an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
, and therefore he gave the product a name that suggested this. By the time he discovered his mistake, the name “glycal” was adopted as a general name for all sugars with a double bond between carbon atoms 1 and 2.
Conformation of Glycals

Pyranose
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
(six-membered) or furanose
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
(five-membered) rings, depending on the monosaccharide used as a starting material to synthesize the glycal. Glycals can also be classified as endo-glycals or exo-glycals. A glycal is an endo-glycal when the double bond is within the ring. If the hydroxyl group on carbon 1 has been replaced with another carbon atom, a double bond can also form outside the ring between carbon 1 and this new carbon. In this case, the product is called an exo-glycal. The glycal conformation that has been studied in most depth is that of the pyranose endo-glycal. The favoured conformation of this glycal is the half-chair, a result which has been confirmed by quantum mechanical calculations.
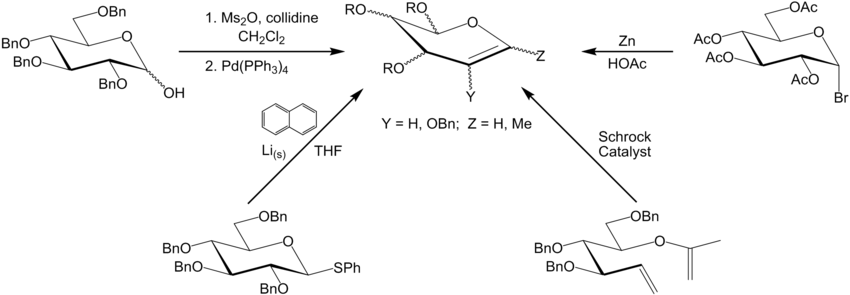
Synthesis of Glycals
The original Fischer glycal synthesis was the reductive elimination with zinc of a glycosyl halide. This glycosyl halide was formed from a monosaccharide starting material. Some other synthetic routes include:- Ring-closing metathesis
- Reaction of thioglycosides with lithium napthalenide.
- Mesylation of the anomeric hydroxyl and formation of the anomeric palladium complex, which undergoes beta-elimination
A general example of each synthetic route is given below (drawn with first discussed synthesis upper right, moving clockwise):
Reactions and Uses of Glycals
The double bond of a glycal allows many other functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s to be introduced into a monosaccharide. Like an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, a glycal can undergo electrophilic addition across the double bond to add in these new atoms such as halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s, epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s, and nitrogen. The glycal double bond also allows a deoxy position (carbon in the ring that doesn’t have an oxygen bonded to it) to be easily introduced.
Glycals have many uses in synthetic carbohydrate chemistry. They are commonly used as glycosylation donors, meaning that they can react with other monosaccharides to form a longer chain of monosaccharides called an oligosaccharide.
Glycals can also have interesting applications in studying biological systems, particularly enzymes. D-glucal and radiolabelled D-galactal have been used to selectively bind with amino acids in the active sites of several enzymes. These enzyme-glycal complexes allow these amino acids that are essential for catalysis to be identified and allow for a better understanding of how these enzymes function.

