
Gibbs isotherm
Encyclopedia
The Gibbs adsorption isotherm for multicomponent systems is an equation used to relate the changes in concentration of a component in contact with a surface with changes in the surface tension
. For a binary system containing two components, the Gibbs adsorption equation in terms of surface excess is:
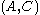
where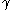 is the surface tension
is the surface tension
, 1 is the surface excess of component 1,
1 is the surface excess of component 1,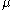 1 is the chemical potential
1 is the chemical potential
of component 1.
. Adsorption influences changes in surface tension
and colloid
stability. Adsorption layers at the surface of a liquid dispersion medium may affect the interactions of the dispersed particles in the media and consequently these layers may play crucial role in colloid stability The adsorption of molecules of liquid phase at an interface occurs when this liquid phase is in contact with other immiscible phases that may be gas, liquid, or solid
describes how difficult it is to extend the area of a surface (by stretching or distorting it). If surface tension is high, there is a large free energy required to increase the surface area, so the surface will tend to contract and hold together like a rubber sheet.
There are various factors affecting surface tension, one of which is that the composition of the surface may be different from the bulk. For example, if water is mixed with a tiny amount of surfactant
s (for example, hand soap), the bulk water may be 99% water molecules and 1% soap molecules, but the topmost surface of the water may be 50% water molecules and 50% soap molecules. In this case, the soap has a large and positive "surface excess". In other examples, the surface excess may be negative: For example, if water is mixed with an inorganic salt like sodium chloride
, the surface of the water is on average less salty and more pure than the bulk average.
Consider again the example of water with a bit of soap. Since the water surface needs to have higher concentration of soap than the bulk, whenever the water's surface area is increased, it is necessary to remove soap molecules from the bulk and add them to the new surface. If the concentration of soap is increased a bit, the soap molecules are more readily available (they have higher chemical potential
), so it is easier to pull them from the bulk in order to create the new surface. Since it is easier to create new surface, the surface tension is lowered. The general principle is:
Next consider the example of water with salt. The water surface is less salty than bulk, so whenever the water's surface area is increased, it is necessary to remove salt molecules from the new surface and push them into bulk. If the concentration of salt is increased a bit (raising the salt's chemical potential
), it becomes harder to push away the salt molecules. Since it is now harder to create the new surface, the surface tension is higher. The general principle is:
The Gibbs isotherm equation gives the exact quantitative relationship for these trends.
and phase . Experimentally, it is difficult to determine the exact structure of an inhomogeneous surface phase that is contact with a bulk liquid phase containing more than one solute. Inhomogeneity of the surface phase is a result of variation of mole ratios. A model proposed by Josiah Willard Gibbs
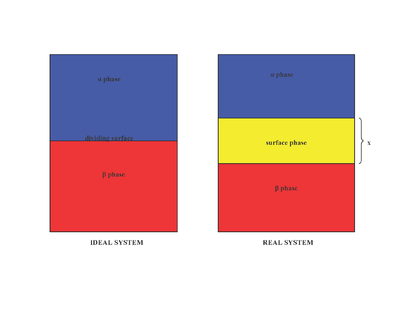
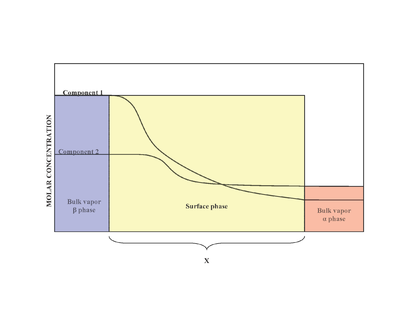
proposed that the surface phase as an idealized model that had zero thickness. In reality, although the bulk regions of and phases are constant, the concentrations of components in the interfacial region will gradually vary from the bulk concentration of to the bulk concentration of over the distance x. This is in contrast to the idealized Gibbs model where the distance x takes on the value of zero. The diagram to the right illustrates the differences between the real and idealized models.
 In the real system, however, the total moles of a component varies depending on the arbitrary placement of the dividing surface. The quantitative measure of adsorption of the -th component is captured by the surface excess quantity. The surface excess represents the difference between the total moles of the -th component in a system and the moles of the -th component in a particular phase (either or ) and is represented by:
In the real system, however, the total moles of a component varies depending on the arbitrary placement of the dividing surface. The quantitative measure of adsorption of the -th component is captured by the surface excess quantity. The surface excess represents the difference between the total moles of the -th component in a system and the moles of the -th component in a particular phase (either or ) and is represented by:
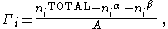
where is the surface excess of the -th component, are the moles, and are the phases, and is the area of the dividing surface.
represents excess of solute per unit area of the surface over what would be present if the bulk concentration prevailed all the way to the surface, it can be positive, negative or zero. It has units of mol/m2.
at the interface to a solvent in the bulk phase. An advantage of using the relative surface excess quantities is that they don't depend on the location of the dividing surface. The relative surface excess of species and solvent 1 is therefore:
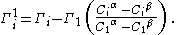
of a system can be written as:
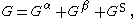
where is the Gibbs free energy.
The equation of the Gibbs Adsorption Isotherm can be derived from the “particularization to the thermodynamics of the Euler theorem on homogeneous first-order forms.” The Gibbs free energy of each phase , phase , and the surface phase can be represented by the equation:
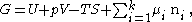
where is the internal energy, is the pressure, is the volume, is the temperature, is the entropy, and is the chemical potential of the -th component.
By taking the total derivative of the Euler form of the Gibbs equation for the phase, phase and the surface phase:
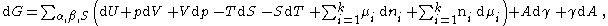
where is the cross sectional area of the diving surface, and is the surface tension
.
For reversible processes, the first law of thermodynamics requires that:
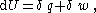
where is the heat energy and is the work.
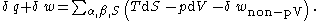
Substituting the above equation into the total derivative of the Gibbs energy equation and by utilizing the result is equated to the non-pressure volume work when surface energy is considered:
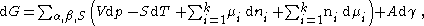
by utilizing the Gibbs-Duhem equation of:

The equation relating the phase, phase and the surface phase becomes:
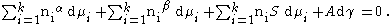
When considering the bulk phases ( phase, phase), at equilibrium at constant temperature and pressure the Gibbs Duhem equation requires that:
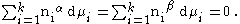
The resulting equation is the Gibbs adsorption isotherm equation:
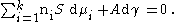
The Gibbs adsorption isotherm is an equation which could be considered an adsorption
isotherm that connects surface tension
of a solution with the concentration of the solute.
For a binary system containing two components the Gibbs Adsorption Equation in terms of surface excess is:
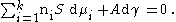
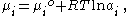
where is the chemical potential
of the -th component, is the chemical potential of the -th component at a reference state, is the gas constant
, is the temperature, and is the activity of the -th component.
Differentiation of the chemical potential equation results in:
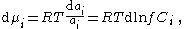
where is the activity coefficient of component , and is the concentration of species in the bulk phase.
If the solutions in the and phases are dilute (rich in one particular component ) then activity coefficient
of the component approaches unity and the Gibbs isotherm becomes:
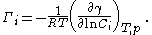
In the derivation of the equation it is assumed that the solution is ideal, (so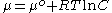 ) and surface concentration of the solvent is zero, so it is only valid under these assumptions. It is also considered that the interface is bidimensional, which is not true, further models, such as Guggenheim's, correct this flaw.
) and surface concentration of the solvent is zero, so it is only valid under these assumptions. It is also considered that the interface is bidimensional, which is not true, further models, such as Guggenheim's, correct this flaw.
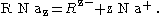
The Gibbs Adsorption equation in terms of the relative surface excess becomes:
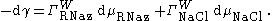
The Relation Between Surface Tension and The Surface Excess Concentration becomes:
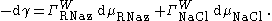
where is the coefficient of the Gibbs adsorption. Values of are calculated using the Double layer (interfacial)
models of Helmholtz, Gouy
, and Stern
.
Substances can have different effects on surface tension as shown :
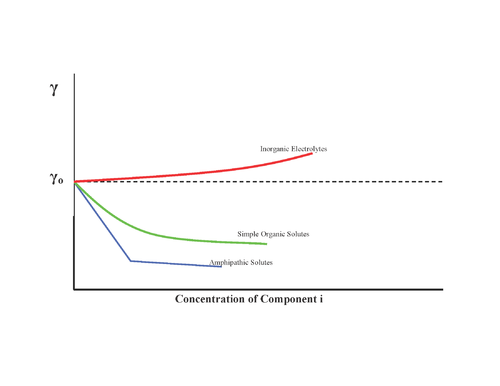
Therefore inorganic salts have negative surface concentrations (which is logical, because they have strong attractions with the solvent) and surfactants have positive surface concentrations: they adsorb on the interface
.
A method for determining surface concentrations is needed in order to prove the validity of the model: two different techniques are normally used: ellipsometry and following the decay of 14C present in the surfactant molecules.

where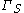 refers to the surface concentration of surfactant molecules, without considering the counter ion.
refers to the surface concentration of surfactant molecules, without considering the counter ion.
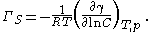

concentration data and the Gibbs adsorption equation. The microtome
blade method is used to determine the weight and molal concentration of an interface. The method involves attaining a one square meter portion of air-liquid interface of binary solutions using a microtome
blade.
Another method that is used to determine the extent of adsorption at a air-water interface is the emulsion technique, which can be used to estimate the relative surface excess with respect to water.
Additionally, the Gibbs surface excess of a surface active component for an aqueous solution can be found using the radioactive tracer
method. The surface active component is usually labeled with carbon-14
or sulfur-35.
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
. For a binary system containing two components, the Gibbs adsorption equation in terms of surface excess is:
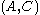
where
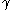 is the surface tension
is the surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
,
 1 is the surface excess of component 1,
1 is the surface excess of component 1,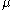 1 is the chemical potential
1 is the chemical potentialChemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of component 1.
Adsorption
Different influences at the interface may cause changes in the composition of the near-surface layer Substances may either accumulate near the surface or conversely, move into the bulk. The movement of the molecules characterizes the phenomena of adsorptionAdsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
. Adsorption influences changes in surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
and colloid
Colloid
A colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
stability. Adsorption layers at the surface of a liquid dispersion medium may affect the interactions of the dispersed particles in the media and consequently these layers may play crucial role in colloid stability The adsorption of molecules of liquid phase at an interface occurs when this liquid phase is in contact with other immiscible phases that may be gas, liquid, or solid
Conceptual explanation of equation
Surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
describes how difficult it is to extend the area of a surface (by stretching or distorting it). If surface tension is high, there is a large free energy required to increase the surface area, so the surface will tend to contract and hold together like a rubber sheet.
There are various factors affecting surface tension, one of which is that the composition of the surface may be different from the bulk. For example, if water is mixed with a tiny amount of surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
s (for example, hand soap), the bulk water may be 99% water molecules and 1% soap molecules, but the topmost surface of the water may be 50% water molecules and 50% soap molecules. In this case, the soap has a large and positive "surface excess". In other examples, the surface excess may be negative: For example, if water is mixed with an inorganic salt like sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
, the surface of the water is on average less salty and more pure than the bulk average.
Consider again the example of water with a bit of soap. Since the water surface needs to have higher concentration of soap than the bulk, whenever the water's surface area is increased, it is necessary to remove soap molecules from the bulk and add them to the new surface. If the concentration of soap is increased a bit, the soap molecules are more readily available (they have higher chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
), so it is easier to pull them from the bulk in order to create the new surface. Since it is easier to create new surface, the surface tension is lowered. The general principle is:
- When the surface excess of a component is positive, increasing the chemical potential of that component reduces the surface tension.
Next consider the example of water with salt. The water surface is less salty than bulk, so whenever the water's surface area is increased, it is necessary to remove salt molecules from the new surface and push them into bulk. If the concentration of salt is increased a bit (raising the salt's chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
), it becomes harder to push away the salt molecules. Since it is now harder to create the new surface, the surface tension is higher. The general principle is:
- When the surface excess of a component is negative, increasing the chemical potential of that component increases the surface tension.
The Gibbs isotherm equation gives the exact quantitative relationship for these trends.
Location of surface and defining surface excess

Location of surface
In the presence of two phases ( and ), the surface (surface phase) is located in between the phasePhase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
and phase . Experimentally, it is difficult to determine the exact structure of an inhomogeneous surface phase that is contact with a bulk liquid phase containing more than one solute. Inhomogeneity of the surface phase is a result of variation of mole ratios. A model proposed by Josiah Willard Gibbs
Josiah Willard Gibbs
Josiah Willard Gibbs was an American theoretical physicist, chemist, and mathematician. He devised much of the theoretical foundation for chemical thermodynamics as well as physical chemistry. As a mathematician, he invented vector analysis . Yale University awarded Gibbs the first American Ph.D...
proposed that the surface phase as an idealized model that had zero thickness. In reality, although the bulk regions of and phases are constant, the concentrations of components in the interfacial region will gradually vary from the bulk concentration of to the bulk concentration of over the distance x. This is in contrast to the idealized Gibbs model where the distance x takes on the value of zero. The diagram to the right illustrates the differences between the real and idealized models.
Definition of surface excess
In the idealized model, the chemical components of the and bulk phases remain unchanged except when approaching the dividing surface. The total moles of any component (Examples include: water, ethylene glycol etc.) remains constant in the bulk phases but varies in the surface phase for the real system model as shown below.
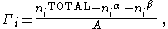
where is the surface excess of the -th component, are the moles, and are the phases, and is the area of the dividing surface.
represents excess of solute per unit area of the surface over what would be present if the bulk concentration prevailed all the way to the surface, it can be positive, negative or zero. It has units of mol/m2.
Relative surface excess
Relative Surface Excess quantities are more useful than arbitrary surface excess quantities. The Relative surface excess relates the adsorptionAdsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
at the interface to a solvent in the bulk phase. An advantage of using the relative surface excess quantities is that they don't depend on the location of the dividing surface. The relative surface excess of species and solvent 1 is therefore:
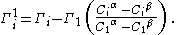
Derivation of the Gibbs adsorption equation
For a two-phase system consisting of the and phase in equilibrium with a surface s dividing the phases, the total Gibbs free energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of a system can be written as:
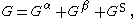
where is the Gibbs free energy.
The equation of the Gibbs Adsorption Isotherm can be derived from the “particularization to the thermodynamics of the Euler theorem on homogeneous first-order forms.” The Gibbs free energy of each phase , phase , and the surface phase can be represented by the equation:
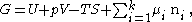
where is the internal energy, is the pressure, is the volume, is the temperature, is the entropy, and is the chemical potential of the -th component.
By taking the total derivative of the Euler form of the Gibbs equation for the phase, phase and the surface phase:
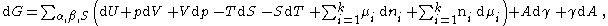
where is the cross sectional area of the diving surface, and is the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
.
For reversible processes, the first law of thermodynamics requires that:
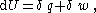
where is the heat energy and is the work.
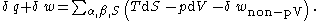
Substituting the above equation into the total derivative of the Gibbs energy equation and by utilizing the result is equated to the non-pressure volume work when surface energy is considered:
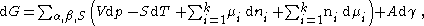
by utilizing the Gibbs-Duhem equation of:

The equation relating the phase, phase and the surface phase becomes:
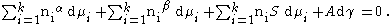
When considering the bulk phases ( phase, phase), at equilibrium at constant temperature and pressure the Gibbs Duhem equation requires that:
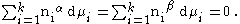
The resulting equation is the Gibbs adsorption isotherm equation:
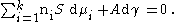
The Gibbs adsorption isotherm is an equation which could be considered an adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
isotherm that connects surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
of a solution with the concentration of the solute.
For a binary system containing two components the Gibbs Adsorption Equation in terms of surface excess is:
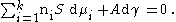
Relation between surface tension and the surface excess concentration
The chemical potential of species in solution depends on the activity a using the following equation: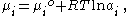
where is the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of the -th component, is the chemical potential of the -th component at a reference state, is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
, is the temperature, and is the activity of the -th component.
Differentiation of the chemical potential equation results in:
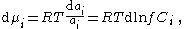
where is the activity coefficient of component , and is the concentration of species in the bulk phase.
If the solutions in the and phases are dilute (rich in one particular component ) then activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
of the component approaches unity and the Gibbs isotherm becomes:
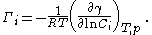
In the derivation of the equation it is assumed that the solution is ideal, (so
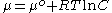 ) and surface concentration of the solvent is zero, so it is only valid under these assumptions. It is also considered that the interface is bidimensional, which is not true, further models, such as Guggenheim's, correct this flaw.
) and surface concentration of the solvent is zero, so it is only valid under these assumptions. It is also considered that the interface is bidimensional, which is not true, further models, such as Guggenheim's, correct this flaw.Gibbs equation for electrolyte adsorption
Consider a system composed of water that contains an organic electrolyte RNaz and an inorganic electrolyte NaCl that both dissociate completely such that: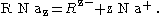
The Gibbs Adsorption equation in terms of the relative surface excess becomes:
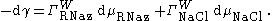
The Relation Between Surface Tension and The Surface Excess Concentration becomes:
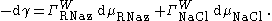
where is the coefficient of the Gibbs adsorption. Values of are calculated using the Double layer (interfacial)
Double layer (interfacial)
A double layer is a structure that appears on the surface of an object when it is placed into a liquid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object...
models of Helmholtz, Gouy
Louis Georges Gouy
Louis Georges Gouy was a French physicist who was born at Vals-les-Bains, Ardèche in 1854 and died January 27 1926. He is the namesake of the Gouy balance, the Gouy-Chapman electric double layer model and the ....
, and Stern
Otto Stern
Otto Stern was a German physicist and Nobel laureate in physics.-Biography:Stern was born in Sohrau, now Żory in the German Empire's Kingdom of Prussia and studied at Breslau, now Wrocław in Lower Silesia....
.
Substances can have different effects on surface tension as shown :

- No effect, for example sugar
- Increase of surface tension, inorganic salts
- Decrease surface tension progressively, alcohols
- Decrease surface tension and, once a minimum is reached, no more effect: surfactants
Therefore inorganic salts have negative surface concentrations (which is logical, because they have strong attractions with the solvent) and surfactants have positive surface concentrations: they adsorb on the interface
Interface (chemistry)
An interface is a surface forming a common boundary among two different phases, such as an insoluble solid and a liquid, two immiscible liquids or a liquid and an insoluble gas. The importance of the interface depends on which type of system is being treated: the bigger the quotient area/volume,...
.
A method for determining surface concentrations is needed in order to prove the validity of the model: two different techniques are normally used: ellipsometry and following the decay of 14C present in the surfactant molecules.
Gibbs isotherm for ionic surfactants
Ionic surfactants require special considerations, as they are electrolytes:- In absence of extra electrolytes

where
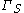 refers to the surface concentration of surfactant molecules, without considering the counter ion.
refers to the surface concentration of surfactant molecules, without considering the counter ion.- In presence of added electrolytes
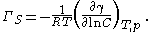

Experimental methods
The extent of adsorption at a liquid interface can be evaluated using the surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
concentration data and the Gibbs adsorption equation. The microtome
Microtome
A microtome is a sectioning instrument that allows for the cutting of extremely thin slices of material, known as sections. Microtomes are an important device in microscopy preparation, allowing for the preparation of samples for observation under transmitted light or electron radiation...
blade method is used to determine the weight and molal concentration of an interface. The method involves attaining a one square meter portion of air-liquid interface of binary solutions using a microtome
Microtome
A microtome is a sectioning instrument that allows for the cutting of extremely thin slices of material, known as sections. Microtomes are an important device in microscopy preparation, allowing for the preparation of samples for observation under transmitted light or electron radiation...
blade.
Another method that is used to determine the extent of adsorption at a air-water interface is the emulsion technique, which can be used to estimate the relative surface excess with respect to water.
Additionally, the Gibbs surface excess of a surface active component for an aqueous solution can be found using the radioactive tracer
Radioactive tracer
A radioactive tracer, also called a radioactive label, is a substance containing a radioisotope that is used to measure the speed of chemical processes and to track the movement of a substance through a natural system such as a cell or tissue...
method. The surface active component is usually labeled with carbon-14
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
or sulfur-35.

