.gif)
Gauche (stereochemistry)
Encyclopedia
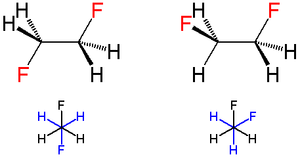
The term "gauche" refers to conformational isomers
(conformers) where two vicinal
groups are separated by a 60° torsion angle. IUPAC defines groups as gauche if they have a "synclinal alignment of groups attached to adjacent atoms".
In stereochemistry
, gauche interactions hinder bond
rotation. For example, sighting along the C2-C3 bond in staggered butane
, there are two possible relative potential energies
. The two methyl groups can be in an anti-bonding relationship, or offset at sixty degree dihedral angles. In the latter configuration, the two methyls are said to be in a gauche relationship, and the relative potential energy of each methyl-methyl gauche interaction is 0.9 kilocalories per mole
(4 kJ/mol). In general a gauche rotamer is less stable than an anti-rotamer.
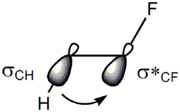 There are two main explanations for the gauche effect: hyperconjugation
There are two main explanations for the gauche effect: hyperconjugation
and bent bonds. In the hyperconjugation model, the donation of electron density from the C–H σ bonding orbital to the C–F σ* antibonding orbital is considered the source of stabilization in the gauche isomer. Due to the greater electronegativity of fluorine, the C–H σ orbital is a better electron donor than the C–F σ orbital, while the C–F σ* orbital is a better electron acceptor than the C–H σ* orbital. Only the gauche conformation allows good overlap between the better donor and the better acceptor.
Key in the bent bond explanation of the gauche effect in difluoroethane is the increased p orbital character of both C-F bonds due to the large electronegativity of fluorine. As a result electron density builds up above and below to the left and right of the central C-C bond. The resulting reduced orbital overlap
can be partially compensated when a gauche conformation is assumed, forming a bent bond. Of these two models, hyperconjugation is generally considered the principal cause behind the gauche effect in difluoroethane.
The molecular geometry
of both rotamers can be obtained experimentally by high resolution infrared spectroscopy
augmented with in silico
work. In accordance with the model described above, the carbon - carbon bond length
is higher for the anti-rotamer (151.4 pm vs. 150). The steric repulsion between the fluorine atoms in the gauche rotamer causes increased CCF bond angles (by 3.2°) and increased FCCF dihedral angle
s (from the default 60° to 71°).
In the related 1,2-difluorodiphenylethane (two protons replaced by phenyl) the threo isomer is found (by X-ray diffraction and from NMR coupling constant
s) to have an anti conformation between the two phenyl groups and the two fluorine
groups and a gauche conformation is found for both groups for the erythro isomer. According to in silico
results this conformation is more stable by 0.21 kcal/mol (880 J/mol).
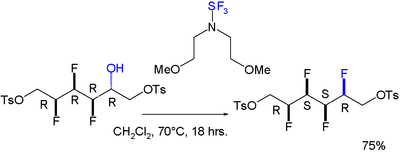
A gauche effect has also been reported for a molecule featuring 4 successive fluor syn substituents, the last one introduced by deoxofluorinating agent bis(2-methoxyethyl)aminosulfur trifluoride :
 The gauche effect is very sensitive to solvent effects, due to the large difference in polarity of the two conformers. For example, 2,3-dinitro-2,3-dimetylbutane, which in the solid state exists only in the gauche conformation, prefers the gauche conformer in benzene
The gauche effect is very sensitive to solvent effects, due to the large difference in polarity of the two conformers. For example, 2,3-dinitro-2,3-dimetylbutane, which in the solid state exists only in the gauche conformation, prefers the gauche conformer in benzene
solution by a ratio of 79:21, but in carbon tetrachloride
it prefers the anti conformer by a ratio of 58:42. Another case is trans-1,2 difluorocyclohexane, which has a larger preference for the eq/eq conformer in more polar solvents.
A related effect is the cis effect in alkenes.
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
(conformers) where two vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
groups are separated by a 60° torsion angle. IUPAC defines groups as gauche if they have a "synclinal alignment of groups attached to adjacent atoms".
In stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
, gauche interactions hinder bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
rotation. For example, sighting along the C2-C3 bond in staggered butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
, there are two possible relative potential energies
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
. The two methyl groups can be in an anti-bonding relationship, or offset at sixty degree dihedral angles. In the latter configuration, the two methyls are said to be in a gauche relationship, and the relative potential energy of each methyl-methyl gauche interaction is 0.9 kilocalories per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(4 kJ/mol). In general a gauche rotamer is less stable than an anti-rotamer.
Gauche effect
The Gauche effect characterizes any gauche rotamer which is actually more stable than the anti rotamer. This effect is present in 1,2-difluoroethane (H2FCCFH2) for which the gauche conformation is more stable by 2.4 to 3.4 kJ/mole in the gas phase. Another example is 1,2-dimethoxyethane.
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
and bent bonds. In the hyperconjugation model, the donation of electron density from the C–H σ bonding orbital to the C–F σ* antibonding orbital is considered the source of stabilization in the gauche isomer. Due to the greater electronegativity of fluorine, the C–H σ orbital is a better electron donor than the C–F σ orbital, while the C–F σ* orbital is a better electron acceptor than the C–H σ* orbital. Only the gauche conformation allows good overlap between the better donor and the better acceptor.
Key in the bent bond explanation of the gauche effect in difluoroethane is the increased p orbital character of both C-F bonds due to the large electronegativity of fluorine. As a result electron density builds up above and below to the left and right of the central C-C bond. The resulting reduced orbital overlap
Orbital overlap
Orbital overlap was an idea first introduced by Linus Pauling to explain the molecular bond angles observed through experimentation and is the basis for the concept of orbital hybridisation. s orbitals are spherical and have no directionality while p orbitals are oriented 90° to one another...
can be partially compensated when a gauche conformation is assumed, forming a bent bond. Of these two models, hyperconjugation is generally considered the principal cause behind the gauche effect in difluoroethane.
The molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
of both rotamers can be obtained experimentally by high resolution infrared spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
augmented with in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
work. In accordance with the model described above, the carbon - carbon bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
is higher for the anti-rotamer (151.4 pm vs. 150). The steric repulsion between the fluorine atoms in the gauche rotamer causes increased CCF bond angles (by 3.2°) and increased FCCF dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s (from the default 60° to 71°).
In the related 1,2-difluorodiphenylethane (two protons replaced by phenyl) the threo isomer is found (by X-ray diffraction and from NMR coupling constant
Coupling constant
In physics, a coupling constant, usually denoted g, is a number that determines the strength of an interaction. Usually the Lagrangian or the Hamiltonian of a system can be separated into a kinetic part and an interaction part...
s) to have an anti conformation between the two phenyl groups and the two fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
groups and a gauche conformation is found for both groups for the erythro isomer. According to in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
results this conformation is more stable by 0.21 kcal/mol (880 J/mol).
A gauche effect has also been reported for a molecule featuring 4 successive fluor syn substituents, the last one introduced by deoxofluorinating agent bis(2-methoxyethyl)aminosulfur trifluoride :

Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
solution by a ratio of 79:21, but in carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
it prefers the anti conformer by a ratio of 58:42. Another case is trans-1,2 difluorocyclohexane, which has a larger preference for the eq/eq conformer in more polar solvents.
A related effect is the cis effect in alkenes.