Anomeric effect
Encyclopedia
In organic chemistry
, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom
within a cyclohexane
ring to prefer the axial orientation instead of the less hindered equatorial orientation that would be expected from steric
considerations. This effect was originally observed in pyranose
rings by J. T. Edward in 1955; at that time, N.-J. Chii and R. U. Lemieux
began to study the anomerization equilibria of the fully acetylated derivatives of several aldohexopyranoses. The term "anomeric effect" was introduced in 1958. The anomeric effect got its name from the term used to designate the C-1 carbon of a pyranose, the anomeric carbon. Isomers that differ only in the configuration at the anomeric carbon are called anomer
s.
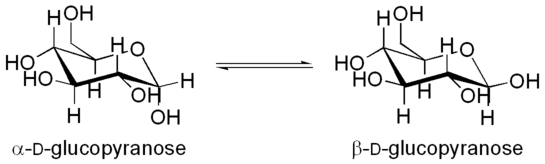
The anomers of glucopyranose are diastereomers, with the beta anomer on the right having an OH group pointing up equatorially in the lower right-hand corner of the figure, and the alpha anomer on the left having that OH group pointing down axially.
The anomeric effect can be generalized to any system with the general formula R–Y–C–Z, where Y is an atom with one or more electronic lone pair
s, and Z is an electronegative atom. The magnitude of the anomeric effect is estimated at about 1–2 kcal/mol in the case of sugars. In this general case, the molecule need not be cyclic. For example, a small molecule that exhibits the anomeric effect and that is often used for theoretical studies is dimethoxymethane
. In the case of dimethoxyethane the gauche,gauche conformation is about 3–5 kcal/mol lower in energy (more stable) than the trans,trans conformation—this is about two times as big as the effect in sugars because there are two rotatable bonds that are affected.
The simplest explanation is that the equatorial configuration has the dipole
s involving both heteroatoms partially aligned, and therefore repelling each other. By contrast the axial configuration has these dipoles roughly opposing, thus representing a more stable and lower energy state.
 In 1998, Box's molecular modeling studies of saccharides, and analysis of crystallographic data of monosaccharides from the Cambridge Crystallographic Database, using the molecular mechanics
In 1998, Box's molecular modeling studies of saccharides, and analysis of crystallographic data of monosaccharides from the Cambridge Crystallographic Database, using the molecular mechanics
based program STR3DI32, resulted in a refinement of this dipolar hypothesis by showing that the dipolar repulsions originally suggested, above, were reinforced by stabilizing, and significant, C-H...O hydrogen bonds involving the acetal functional group. More recent MO calculations are consistent with this hypothesis. This more comprehensive analysis of the origins of the anomeric effect has also resulted in a better understanding of the related, and equally puzzling, reverse anomeric effect.
An alternative and widely accepted explanation is that there is a stabilizing interaction (hyperconjugation
) between the unshared electron pair on the one heteroatom (the endocyclic one in a sugar ring) and the σ* orbital for the axial (exocyclic) C–X bond.
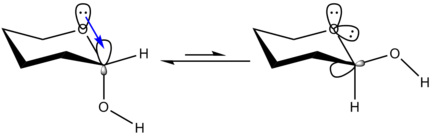 When the exocyclic (in a sugar) atom bears a lone pair of electrons there should also be a similar interaction between that unshared electron pair (of this exocyclic atom) and the σ* orbital of the annular C-O bond. This second interaction, which is a strong feature of the β-anomer (equatorial exocyclic group), should significantly stabilize the β-anomer, and should significantly attenuate the anomeric effect. Thus one would expect that molecules like the glycosyl halides should show small anomeric effects, and have α- and β-anomers of comparable energies. However, it is well known that when the exocyclic atoms bear lone pairs of electrons, the anomeric effect is maximal. Thus, the hyperconjugation hypothesis might contribute to the anomeric effect, but is not the only, or the dominant, stereo-electronic participant.
When the exocyclic (in a sugar) atom bears a lone pair of electrons there should also be a similar interaction between that unshared electron pair (of this exocyclic atom) and the σ* orbital of the annular C-O bond. This second interaction, which is a strong feature of the β-anomer (equatorial exocyclic group), should significantly stabilize the β-anomer, and should significantly attenuate the anomeric effect. Thus one would expect that molecules like the glycosyl halides should show small anomeric effects, and have α- and β-anomers of comparable energies. However, it is well known that when the exocyclic atoms bear lone pairs of electrons, the anomeric effect is maximal. Thus, the hyperconjugation hypothesis might contribute to the anomeric effect, but is not the only, or the dominant, stereo-electronic participant.
Some authors also question the validity of this hyperconjugation model based on results from the theory of atoms in molecules.
While most studies on the anomeric effects have been theoretical in nature, the n–σ* (hyperconjugation) hypothesis has also been extensively criticized on the basis that the electron density redistribution in acetals proposed by this hypothesis, is not congruent with the known experimental chemistry of acetals, and, in particular, the chemistry of monosaccharides.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
within a cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
ring to prefer the axial orientation instead of the less hindered equatorial orientation that would be expected from steric
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
considerations. This effect was originally observed in pyranose
Pyranose
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
rings by J. T. Edward in 1955; at that time, N.-J. Chii and R. U. Lemieux
Raymond U. Lemieux
Raymond Urgel Lemieux, CC, AOE, FRS was a Canadian organic chemist, who pioneered a number of discoveries in the field of chemistry, his first and most famous being the synthesis of sucrose...
began to study the anomerization equilibria of the fully acetylated derivatives of several aldohexopyranoses. The term "anomeric effect" was introduced in 1958. The anomeric effect got its name from the term used to designate the C-1 carbon of a pyranose, the anomeric carbon. Isomers that differ only in the configuration at the anomeric carbon are called anomer
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
s.
The anomers of glucopyranose are diastereomers, with the beta anomer on the right having an OH group pointing up equatorially in the lower right-hand corner of the figure, and the alpha anomer on the left having that OH group pointing down axially.
The anomeric effect can be generalized to any system with the general formula R–Y–C–Z, where Y is an atom with one or more electronic lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s, and Z is an electronegative atom. The magnitude of the anomeric effect is estimated at about 1–2 kcal/mol in the case of sugars. In this general case, the molecule need not be cyclic. For example, a small molecule that exhibits the anomeric effect and that is often used for theoretical studies is dimethoxymethane
Dimethoxymethane
Dimethoxymethane, also called methylal, is a clear colorless flammable liquid with a low boiling point, low viscosity and an excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal of formaldehyde...
. In the case of dimethoxyethane the gauche,gauche conformation is about 3–5 kcal/mol lower in energy (more stable) than the trans,trans conformation—this is about two times as big as the effect in sugars because there are two rotatable bonds that are affected.
Physical origins
Several explanations for the anomeric effect have been proposed.The simplest explanation is that the equatorial configuration has the dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
s involving both heteroatoms partially aligned, and therefore repelling each other. By contrast the axial configuration has these dipoles roughly opposing, thus representing a more stable and lower energy state.

Molecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
based program STR3DI32, resulted in a refinement of this dipolar hypothesis by showing that the dipolar repulsions originally suggested, above, were reinforced by stabilizing, and significant, C-H...O hydrogen bonds involving the acetal functional group. More recent MO calculations are consistent with this hypothesis. This more comprehensive analysis of the origins of the anomeric effect has also resulted in a better understanding of the related, and equally puzzling, reverse anomeric effect.
An alternative and widely accepted explanation is that there is a stabilizing interaction (hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
) between the unshared electron pair on the one heteroatom (the endocyclic one in a sugar ring) and the σ* orbital for the axial (exocyclic) C–X bond.

Some authors also question the validity of this hyperconjugation model based on results from the theory of atoms in molecules.
While most studies on the anomeric effects have been theoretical in nature, the n–σ* (hyperconjugation) hypothesis has also been extensively criticized on the basis that the electron density redistribution in acetals proposed by this hypothesis, is not congruent with the known experimental chemistry of acetals, and, in particular, the chemistry of monosaccharides.