
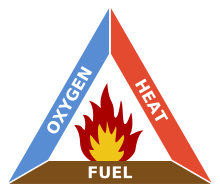
Fire triangle
Encyclopedia
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
s.
The triangle illustrates a fire requires three elements: heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
, and an oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
(usually oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
). The fire is prevented or extinguished by removing any one of them. A fire naturally occurs when the elements are combined in the right mixture.
- Without sufficient heat, a fire cannot begin, and it cannot continue. Heat can be removed by the application of a substance which reduces the amount of heat available to the fire reaction. This is often water, which requires heat for phase change from water to steam. Introducing sufficient quantities and types of powder or gas in the flame reduces the amount of heat available for the fire reaction in the same manner. Scraping emberEmberEmbers are the glowing, hot coals made of greatly heated wood, coal, or other carbon-based material that remain after, or sometimes precede a fire. Embers can glow very hot, sometimes as hot as the fire which created them...
s from a burning structure also removes the heat source. Turning off the electricity in an electrical fire removes the ignition source.
- Without fuel, a fire will stop. Fuel can be removed naturally, as where the fire has consumed all the burnable fuel, or manually, by mechanically or chemically removing the fuel from the fire. Fuel separation is an important factor in wildland fireWildfireA wildfire is any uncontrolled fire in combustible vegetation that occurs in the countryside or a wilderness area. Other names such as brush fire, bushfire, forest fire, desert fire, grass fire, hill fire, squirrel fire, vegetation fire, veldfire, and wilkjjofire may be used to describe the same...
suppression, and is the basis for most major tactics, such as controlled burnControlled burnControlled or prescribed burning, also known as hazard reduction burning or Swailing is a technique sometimes used in forest management, farming, prairie restoration or greenhouse gas abatement. Fire is a natural part of both forest and grassland ecology and controlled fire can be a tool for...
s. The fire stops because a lower concentration of fuel vapor in the flame leads to a decrease in energy release and a lower temperature. Removing the fuel thereby decreases the heat.
- Without sufficient oxygen, a fire cannot begin, and it cannot continue. With a decreased oxygen concentration, the combustion process slows. In most cases, there is plenty of air left when the fire goes out so this is commonly not a major factor.
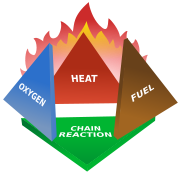
Fire tetrahedron
Halon
Halon can refer to:* Haloalkane, or halogenoalkane, a group of chemical compounds consisting of alkanes with linked halogens. In particular, bromine-containing haloalkanes.* Halomethane fire extinguishing systems...
is due to its interference in the fire chemical reaction.
Combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
is the chemical reaction that feeds a fire more heat and allows it to continue. When the fire involves burning metals like lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
, magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, etc. (known as a class-D fire), it becomes even more important to consider the energy release. The metals react faster with water than with oxygen and thereby more energy is released. Putting water on such a fire results in the fire getting hotter or even exploding
Explosion
An explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"...
because the metals react with water in an exothermic reaction
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
. Carbon dioxide extinguishers are ineffective against certain metals such as titanium. Therefore, inert agents (e.g. dry sand) must be used to break the chain reaction of metallic combustion.
In the same way, as soon as we remove one out of the 3 elements of the triangle, combustion stops.
Fire classes
Based on the combustible material involved, the fire can be classified. In the European Standard "Classification of fires" (EN 2:1992, incorporatiing amendment A1:2004), the fires are classified as:- Class A fire: Ordinary combustibles such as wood, paper, carton, textile, and PVC;
- Class B fire: Flammable liquids and solids which can take a liquid form, such as benzene, gasoline, oil;
- Class C fire: Flammable gases, such as butane, propane, and natural gas;
- Class D fire: Combustible metals, such as iron, aluminum, sodium, and magnesium;
- Class F fire: Cooking media, such as oils and fats, in cooking appliances;
A fire involving energized electrical equipment is not classified by its electrical property.
In the American standard, fires are classified as:
- Class A fire: Ordinary combustibles such as wood, paper, carton, textile, and PVC;
- Class B fire: Flammable liquid or gaseous fuels such benzene, gasoline, oil, butane, propane, and natural gas;
- Class C fire: Involving energized electrical equipment, often caused by short circuits or overheated electrical cables;
- Class D fire: Combustible metals, such as iron, aluminum, sodium, and magnesium;
- Class K fire: Containing a fat element, such as cooking oil
Oxidizer
The oxidizer is the other reactive of the chemical reaction. In most cases, it is the ambient air, and in particular one of its components, Oxygen (O2). By depriving a fire of air, we extinguish it; for example, when covering the flame of a small candle with an empty glass, fire stops; to the contrary, if we blow over a wood fire, we activate it (by bringing more air). In certain torches, we bring dioxygen to improve combustion.In certain cases such as some explosives, the oxidizer and combustible are the same (e.g., nitroglycerin, an unstable molecule that has oxidizing parts in the same molecule as the oxidizeable parts).
Reaction is initiated by an activating energy, in most cases, it is heat. Several examples include friction, as in case of matches, heating an electrical wire, a flame (propagation of fire), or a spark (from a lighter or from any starting electrical device). There are also many other ways to bring sufficient activation energy including electricity, radiation, and pressure, all of which will lead to a temperature rise. In most cases, heat production enables self-sustainability of the reaction, and enables a chain reaction to grow. The temperature at which a liquid produces sufficient vapor to get a flammable mix with self-sustainable combustion is called its flash-point.
Extinction of the fire
To stop a combustion reaction, one of the three elements of the fire-triangle has to be removed:- Suppression of the Combustible: by closing of the valve fueling the combustion, creating sufficient distance between fire and flame, exhausting hot smoke (containing unburned elements), …
- Suppression of the Oxidizer (also known as choking): by the use of a carbon dioxide fire-extinguisher, a blanket, or spraying sufficient water on a solid combustible (water vapor removes fresh air) …
- Suppression of the Activation Energy (cooling down): by spraying water in a mix of air + combustible particles), net absorbing the heat ("Davy" miner lamp), exhausting to remove hot smoke, …
Role of water in fire-fighting
Water can have two different roles:1. In the case of a solid combustible, the solid fuel produce pyrolyzing products under the influence of heat, commonly radiation. This process is halted by the application of water, since water is more easily evaporated than the fuel is pyrolyzed. Thereby energy is removed from the fuel surface and it is cooled and the pyrolyze is stopped, removing the fuel supply to the flames. In fire fighting, this is referred to as surface cooling
2. In the gas phase, i.e. in the flames or in the smoke, the combustible can not be separated from the oxidizer, the only possible action consists of cooling down. In this case, water droplets are evaporated in the gas phase, thereby lowering the temperature and adding water vapour making the gas mixture non combustible. This requires droplets of a size less than about 0.2 mm. In fire fighting, this is referred to as gas cooling or smoke cooling.
There also exist cases where the ignition factor is not the activation energy. For example, smoke explosion is a very violent combustion of unburned gases contained in the smoke created by a sudden fresh air input (oxidizer input). The interval in which an air/gas mix can burn is limited by the explosive limits of the air. This interval can be very small (kerosene) or large (acetylene).
Role of water additives
The role of water in extinguishing a fire can be summarized as follows: The main effect is cooling down the fire by absorption of heat energy either at the fuel surface or in the gas phase. A contributing effect is diluting the atmosphere by adding vapor and thereby removing oxygen from the fireThe main limits to the use of water are directly linked to the physical-chemical characteristics of water itself:
- Water can’t be used on certain type of fires :
- Electrical fires (C class) - as water conducts electricity it presents an electrocution hazard
- Hydrocarbon fires (B class) - as it will only spread the fire because of the difference in density
- Metal fires (D class) - as these fires produce huge amounts of energy (up to 7.550 calories/kg for Aluminum) and water can also create violent chemical reaction with burning metal (by oxidization)
- Fat fires (F class) - as vapor will carry and spread burning oil everywhere.
Since these reactions are well-understood, it has been possible to create specific water-additives which will allow:
- A better heat absorption with a higher density than water
- Carrying free radical catchers on the fire
- Carrying foaming agents to enable water to stay on the surface of a liquid fire and prevent gas release
- Carrying specific reactives which will react and change the nature of the burning material
Water-additives are generally designed to be effective on several categories of fires (class A + class B or even class A + class B + class F), meaning a better global performance and polyvalence of the fire-extinguisher.
Chemistry of Combustion
Combustion is a chemical reaction in which complex molecules are broken down into smaller, more stable molecules through a rearrangement of atomic bonds. A major component of the chemistry of high-temperature combustion involves radical reactions. However, it is possible to consider combustion as a single overall reaction.Example :
H3C-CH2-CH3 + 5O2 → 3CO2 + 4H2O
Carbon dioxide and water are more stable than oxygen and propane.
Combustion is an oxidation-reduction reaction, meaning oxidization of a combustible by an oxidizer;
• combustible is being oxidized during combustion, it is a reducer as it loses electrons;
• Oxidizer is the part being reduced; it is an oxidizer as it gains electrons.
As with any chemical reaction, a catalyst encourages combustion and as it has a high activation energy level, the use of a catalyst enables working at lower temperature. This leads to more complete combustion as in the catalyst of the exhaust of a car, where catalytic metals burn residues contained in the exhaust smoke at lower temperature than in the engine.
Concerning solid combustible, the activation energy allows for vaporization or pyrolysis of the combustible. Gas produced will then mix with an oxidizer resulting in a combustible mixture. If the energy produced by the combustion is higher or equal to the quantity of energy required for the combustion, the reaction is then self-sustainable.
Produced energy and calorific power
The quantity of energy produced by the reaction is higher than the quantity of energy requested to start it.The quantity of energy produced by the combustion is given in Joules (J); it is the enthalpy of the reaction. In the application domains (oven, burner, engine with internal combustion, fire-fighting), we use the notion of calorific power, what is basically the enthalpy of the chemical reaction per unit of weight of combustible or the obtained energy given by the combustion of one kilogram of combustible, expressed in kilojoules per kilogram (kJ/kg or kJ•kg-1).
Combustion of hydrocarbon produces water in its vapor form. ; This water vapor contains huge amount of energy and this parameter has to be taken into account in a specific way to evaluate correctly the calorific power. We define:
• Superior Calorific Power (SCP): « Quantity of energy produced during a complete combustion of a combustible unit, water vapor is said condensed and heat collected »2.
• Inferior Calorific Power (ICP): « Quantity of energy produced during a complete combustion of a combustible unit, Water vapor is said non-condensed and heat not collected »3.
Difference between ICP and SCP is the latent heat of water vaporization (Lv) multiplied by the quantity of produced vapor (m), what equals +/- 2 250 kJ•kg-1 (this value is influenced by pressure and temperature).
We have the relation SCP = ICP + m•Lv.
See also
- Carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
- CombustionCombustionCombustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
- Glossary of firefighting termsGlossary of firefighting termsFirefighting jargon includes a diverse lexicon of both common and idiosyncratic terms. One problem that exists in trying to create a list such as this is that much of the terminology used by a particular department is specifically defined in their particular standing operating procedures, such that...
- Inert gasInert gasAn inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
- OxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
- Fire classesFire classesIn firefighting, fires are identified according to one or more fire classes. Each class designates the fuel involved in the fire, and thus the most appropriate extinguishing agent. The classifications allow selection of extinguishing agents along lines of effectiveness at putting the type of fire...

