Electrical resistance heating remediation
Encyclopedia
Electrical Resistance Heating (ERH) is an intensive in situ environmental remediation method that uses the flow of alternating current
electricity to heat soil and groundwater and evaporate contaminants. Electric current is passed through a targeted soil volume between subsurface electrode elements. The resistance to electrical flow that exists in the soil causes the formation of heat; resulting in an increase in temperature until the boiling point of water at depth is reached. After reaching this temperature, further energy input causes a phase change, forming steam and removing volatile contaminants. ERH is typically more cost effective when used for treating contaminant source areas.
Six-phase heating (see Technology below) was created and patented for the US Department of Energy (DOE) in the 1980s for use on DOE sites as well as commercial applications.
, the air, steam and volatilized contaminants are then treated at the surface to separate water, air and the contaminants. Treatment of the various streams depends on local regulations and the amount of contaminant.
Some low volatility organic contaminants have a short hydrolysis
half life. For contaminants like these, i.e. 1,1,2,2-Tetrachloroethane
and 1,1,1-trichloroethane
, hydrolysis can be the primary form of remediation. As the subsurface is heated the hydrolysis half life of the contaminant will decrease as described by the Arrhenius equation
. This results in a rapid degradation of the contaminant. The hydrolysis by-product
may be remediated by conventional ERH, however the majority of the mass of the primary contaminant will not be recovered but rather will degrade to a by-product.
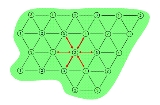
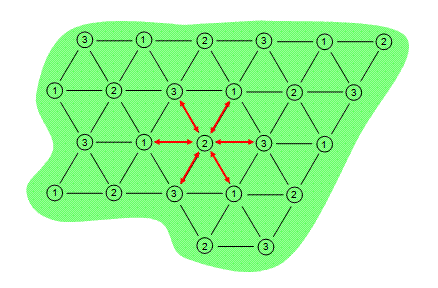
There are predominantly two electrical load arrangements for ERH: three-phase and six-phase. Three-phase heating consists of electrodes in a repeating triangular or delta pattern. Adjacent electrodes are of a different electrical phase
so electricity conducts between them as shown in Figure 1. The contaminated area is depicted by the green shape while the electrodes are depicted by the numbered circles.
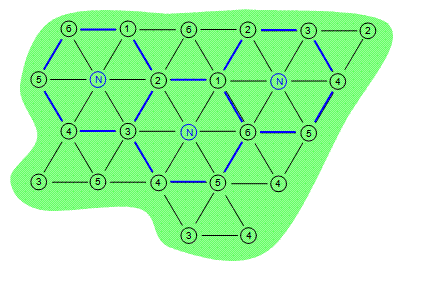
 Six-phase heating consists of six electrodes in a hexagonal pattern with a neutral electrode in the center of the array. The six-phase arrays are outlined in blue in Figure 2 below. Once again the contaminated area is depicted by the green shape while the electrodes are depicted by the numbered circles. In a six-phase heating pattern there can be hot spots and cold spots depending on the phases that are next to each other. For this reason, six-phase heating typically works best on small circular areas that are less than 65 feet in diameter.
Six-phase heating consists of six electrodes in a hexagonal pattern with a neutral electrode in the center of the array. The six-phase arrays are outlined in blue in Figure 2 below. Once again the contaminated area is depicted by the green shape while the electrodes are depicted by the numbered circles. In a six-phase heating pattern there can be hot spots and cold spots depending on the phases that are next to each other. For this reason, six-phase heating typically works best on small circular areas that are less than 65 feet in diameter.
 ERH is typically most effective on volatile organic compounds (VOCs). The chlorinated compounds perchloroethylene, trichloroethylene, and cis or trans 1,2-dichloroethylene are contaminants that are easily remediated with ERH. The table shows contaminants that can be remediated with ERH along with their respective boiling points. Less volatile contaminants like xylene or diesel can also be remediated with ERH but energy requirements increase as the volatility decreases.
ERH is typically most effective on volatile organic compounds (VOCs). The chlorinated compounds perchloroethylene, trichloroethylene, and cis or trans 1,2-dichloroethylene are contaminants that are easily remediated with ERH. The table shows contaminants that can be remediated with ERH along with their respective boiling points. Less volatile contaminants like xylene or diesel can also be remediated with ERH but energy requirements increase as the volatility decreases.
Electrode spacing and operating time can be adjusted to balance the overall remediation cost with the desired cleanup time. A typical remediation may consist of electrodes spaced 15 to 20 feet apart with operating times usually less than a year. The design and cost of an ERH remediation system depends on a number of factors, primarily the volume of soil/groundwater to be treated, the type of contamination, and the treatment goals. The physical and chemical properties of the target compounds are governed by laws that make heated remediations advantageous over most conventional methods. The electrical energy usage required for heating the subsurface and volatilizing the contaminants can account for 5 to 40% of the overall remediation cost.
There are several laws that govern an ERH remediation. Dalton’s law governs the boiling point of a relatively insoluble contaminant. Raoult’s law governs the boiling point of mutually soluble co-contaminants and Henry’s law governs the ratio of the contaminant in the vapor phase to the contaminant in the liquid phase.
Dalton's Law
For mutually insoluble compounds Dalton’s Law states that the partial pressure of a non aqueous phase liquid (NAPL) is equal to its vapor pressure, and that the NAPL
in contact with water will boil when the vapor pressure of water plus the vapor pressure of the VOC is equal to ambient pressure. When a VOC-steam bubble is formed the composition of the bubble is proportional to the composite’s respective vapor pressures.
Raoult's Law
For mutually soluble compounds, Raoult’s Law states that the partial pressure of a compound is equal to its vapor pressure times its mole fraction. This means that mutually soluble contaminants will volatilize slower than if there was only one compound present.
Henry's Law
Henry’s law describes the tendency of a compound to join air in the vapor phase or dissolve in water. The Henry’s Law constant, sometimes called coefficient, is specific to each compound, varies with temperature, and predicts the amount of contaminant that will stay in the vapor phase or transfer to the liquid phase when exiting the condenser.
Alternating current
In alternating current the movement of electric charge periodically reverses direction. In direct current , the flow of electric charge is only in one direction....
electricity to heat soil and groundwater and evaporate contaminants. Electric current is passed through a targeted soil volume between subsurface electrode elements. The resistance to electrical flow that exists in the soil causes the formation of heat; resulting in an increase in temperature until the boiling point of water at depth is reached. After reaching this temperature, further energy input causes a phase change, forming steam and removing volatile contaminants. ERH is typically more cost effective when used for treating contaminant source areas.
History
Three-phase heating (see Technology below) was originally created to enhance oil recovery. This design was patented in 1976 by Bill Pritchett of ARCO. The patent has expired and is now available for public use.Six-phase heating (see Technology below) was created and patented for the US Department of Energy (DOE) in the 1980s for use on DOE sites as well as commercial applications.
Technology
Electrical resistance heating is used by the environmental restoration industry for remediation of contaminated soil and groundwater. ERH consists of constructing electrodes in the ground, applying alternating current (AC) electricity to the electrodes and heating the subsurface to temperatures that promote the evaporation of contaminants. Volatilized contaminants are captured by a subsurface vapor recovery system and conveyed to the surface along with recovered air and steam. Similar to Soil vapor extractionSoil vapor extraction
Soil Vapor Extraction is an in situ process for soil remediation where contamination is removed from soil by carrying it out through a medium such as air or steam. The extracted soil vapors are separated into liquids and vapors, and each stream is treated as necessary...
, the air, steam and volatilized contaminants are then treated at the surface to separate water, air and the contaminants. Treatment of the various streams depends on local regulations and the amount of contaminant.
Some low volatility organic contaminants have a short hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
half life. For contaminants like these, i.e. 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon. As a refrigerant, it is used under the name R-130....
and 1,1,1-trichloroethane
1,1,1-Trichloroethane
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent...
, hydrolysis can be the primary form of remediation. As the subsurface is heated the hydrolysis half life of the contaminant will decrease as described by the Arrhenius equation
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
. This results in a rapid degradation of the contaminant. The hydrolysis by-product
By-product
A by-product is a secondary product derived from a manufacturing process or chemical reaction. It is not the primary product or service being produced.A by-product can be useful and marketable or it can be considered waste....
may be remediated by conventional ERH, however the majority of the mass of the primary contaminant will not be recovered but rather will degrade to a by-product.
There are predominantly two electrical load arrangements for ERH: three-phase and six-phase. Three-phase heating consists of electrodes in a repeating triangular or delta pattern. Adjacent electrodes are of a different electrical phase
Polyphase system
A polyphase system is a means of distributing alternating current electrical power. Polyphase systems have three or more energized electrical conductors carrying alternating currents with a definite time offset between the voltage waves in each conductor. Polyphase systems are particularly useful...
so electricity conducts between them as shown in Figure 1. The contaminated area is depicted by the green shape while the electrodes are depicted by the numbered circles.


| List of compounds that can be remediated with ERH | ||
| Chemical | Molecular Weight (g) | Boiling Point (°C) |
|---|---|---|
| 1,1,1-trichloroethane 1,1,1-Trichloroethane The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent... |
133.4 | 74 |
| 1,1,2-trichloroethane 1,1,2-Trichloroethane 1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula C2H3Cl3. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents... |
133.4 | 114 |
| 1,1-dichloroethane 1,1-Dichloroethane 1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.... |
99 | 57 |
| 1,1-dichloroethene 1,1-Dichloroethene 1,1-Dichloroethene, commonly called 1,1-dichloroethylene or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents... |
97 | 32 |
| 1,2-dichloroethane 1,2-Dichloroethane The chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour... |
99 | 84 |
| 1,2-dichloropropane 1,2-Dichloropropane 1,2-Dichloropropane is an organic compound classified as a chlorocarbon. It is a colorless, flammable liquid with a chloroform-like odor.1,2-Dichloropropane is used as a chemical intermediate in the production of perchloroethylene and other chlorinated chemicals.In the past, 1,2-dichloropropane... |
167.9 | 97 |
| benzene Benzene Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6.... |
78.1 | 80 |
| carbon tetrachloride Carbon tetrachloride Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent... |
153.8 | 77 |
| chlorobenzene Chlorobenzene Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.-Uses:... |
112.6 | 132 |
| chloroform Chloroform Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous... |
119.4 | 62 |
| cis-1,2-dichloroethyene | 97 | 60 |
| dibromoethane Dibromoethane Dibromoethane can refer to either of two isomeric organobromides with the molecuar formula C2H4Br2:* 1,1-Dibromoethane * 1,2-Dibromoethane See also dibromoethene.... |
187.9 | 132 |
| ethylbenzene Ethylbenzene Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material.... |
106.2 | 136 |
| 1,1,2-Trichloro-1,2,2-trifluoroethane 1,1,2-Trichloro-1,2,2-trifluoroethane Trichlorotrifluoroethane, also called 1,1,2-Trichloro-1,2,2-trifluoroethane or CFC-113 is a chlorofluorocarbon. It has the formula CCl2FC-ClF2.... |
187.4 | 48 |
| gasoline Gasoline Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain... |
100 | 100 |
| methylene chloride/dichloromethane Dichloromethane Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents... |
84.9 | 41 |
| 4-methyl-2-pentanone/methyl isobutyl ketone Methyl isobutyl ketone Methyl isobutyl ketone is the organic compound with the formula 2CHCH2CCH3. This colourless liquid, a ketone, is widely used as a solvent.-Production:... |
100.2 | 117 |
| 2-methoxy-2-methylpropane/methyl tert-butyl ether | 88.1 | 55 |
| perchloroethylene | 165.8 | 121 |
| trichloroethene | 131.5 | 87 |
| tert-butyl alcohol Tert-Butanol tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether... |
74.1 | 83 |
| toluene Toluene Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic... |
92.1 | 111 |
| trans-1,2-dichloroethene 1,2-Dichloroethene 1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a highly flammable, colorless liquid with a sharp, harsh odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene,... |
97 | 48 |
| vinyl chloride Vinyl chloride Vinyl chloride is the organochloride with the formula H2C:CHCl. It is also called vinyl chloride monomer, VCM or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride . At ambient pressure and temperature, vinyl chloride... |
62.5 | -14 |
| xylene Xylene Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached... |
106.2 | 140 |
Electrode spacing and operating time can be adjusted to balance the overall remediation cost with the desired cleanup time. A typical remediation may consist of electrodes spaced 15 to 20 feet apart with operating times usually less than a year. The design and cost of an ERH remediation system depends on a number of factors, primarily the volume of soil/groundwater to be treated, the type of contamination, and the treatment goals. The physical and chemical properties of the target compounds are governed by laws that make heated remediations advantageous over most conventional methods. The electrical energy usage required for heating the subsurface and volatilizing the contaminants can account for 5 to 40% of the overall remediation cost.
There are several laws that govern an ERH remediation. Dalton’s law governs the boiling point of a relatively insoluble contaminant. Raoult’s law governs the boiling point of mutually soluble co-contaminants and Henry’s law governs the ratio of the contaminant in the vapor phase to the contaminant in the liquid phase.
Dalton's Law
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
For mutually insoluble compounds Dalton’s Law states that the partial pressure of a non aqueous phase liquid (NAPL) is equal to its vapor pressure, and that the NAPL
DNAPL
A dense non-aqueous phase liquid or DNAPL is a liquid that is both denser than water and is immiscible in or does not dissolve in water.The term DNAPL is used primarily by environmental engineers and hydrogeologists to describe contaminants in groundwater, surface water and sediments...
in contact with water will boil when the vapor pressure of water plus the vapor pressure of the VOC is equal to ambient pressure. When a VOC-steam bubble is formed the composition of the bubble is proportional to the composite’s respective vapor pressures.
Raoult's Law
For mutually soluble compounds, Raoult’s Law states that the partial pressure of a compound is equal to its vapor pressure times its mole fraction. This means that mutually soluble contaminants will volatilize slower than if there was only one compound present.
Henry's Law
Henry's law
In physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
Henry’s law describes the tendency of a compound to join air in the vapor phase or dissolve in water. The Henry’s Law constant, sometimes called coefficient, is specific to each compound, varies with temperature, and predicts the amount of contaminant that will stay in the vapor phase or transfer to the liquid phase when exiting the condenser.
Weaknesses
- Weaknesses of ERH include heat losses on small sites. Treatment volumes that have a large surface area but are thin with respect to depth will have significant heat losses which makes ERH less efficient. The minimum treatment interval for efficient ERH remediation is approximately 10 vertical feet.
- Co-contaminants like oil or grease make remediation more difficult. Oil and grease cause a Raoult’s Law effect which requires more energy to remove the contaminants.
- Peat or high organic carbon in the subsurface will preferentially adsorb VOCs due to van der WaalsVan der Waals forceIn physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
forces. This preferential adsorption will increase the amount of energy required to remove the VOCs from the subsurface.
- Fuel sites are less-commonly treated by ERH because other less-expensive remediation technologies are available and because fuel sites are usually thin (resulting in significant heat losses).
- Sites within landfills are also challenging because metallic debris can distort the electric current paths. ERH is more uniform in natural soil or rock.
Strengths
- ERH is adaptable to all soil types and sedimentary bedrock. ERH is also effective in both the vadose and saturated zones. Certain lithologiesLithologyThe lithology of a rock unit is a description of its physical characteristics visible at outcrop, in hand or core samples or with low magnification microscopy, such as colour, texture, grain size, or composition. It may be either a detailed description of these characteristics or be a summary of...
can limit traditional methods of remediation by preventing a reliable removal/destruction pathway for the contamination of concern. Because electricity can and does travel through any lithology that contains some water, ERH can be effective in any soil type. By forming buoyant steam bubbles during the heating process, ERH creates a carrier gas that transports the contamination of concern up and out of any soil type. ERH is not capable of desiccating the subsurface. In order for the subsurface to conduct electricity, there must be water present in the subsurface. Conductivity will cease before the subsurface is desiccated.
- ERH is commonly applied under active buildings or manufacturing facilities. Electrodes can be installed above grade within a fenced area or below grade to allow for unrestricted surface access to the treatment area.
- Although principally used for contaminant source areas, ERH can be used to achieve low remedial goals such as maximum contaminant levels, MCLsMaximum Contaminant LevelMaximum Contaminant Levels are standards that are set by the United States Environmental Protection Agency for drinking water quality. An MCL is the legal threshold limit on the amount of a substance that is allowed in public water systems under the Safe Drinking Water Act...
, for drinking water.
- After ERH treatment, elevated subsurface temperatures will slowly cool over a period of months or years and return to ambient. This period with elevated temperatures is an important part of the remediation process. The elevated temperatures will enhance BioremediationBioremediationBioremediation is the use of microorganism metabolism to remove pollutants. Technologies can be generally classified as in situ or ex situ. In situ bioremediation involves treating the contaminated material at the site, while ex situ involves the removal of the contaminated material to be treated...
, hydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
and iron reductive dehalogenation.
External links
- ERH Case Study - Portland, Oregon
- CLU-IN Technology Focus
- ERH in Waukegan, Illinois - Federal Remediation Technologies Roundtable
- Six-Phase Heating Enhancement in Alaska - Federal Remediation Technologies Roundtable
- ERH in Portland, Oregon - Federal Remediation Technologies Roundtable
- ERH in Skokie, Illinois - Federal Remediation Technologies Roundtable
- Technology News and Trends - July 2005
- ERH Technology for MTBE and BTEX Cleanup in Montana
- NAVFAC Cost and Performance Review for ERH
- CLU-IN Technology News and Trends - December 2004

