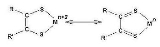
Dithiolene
Encyclopedia
Metal dithiolene complexes are complexes containing dithiolene ligands. Dithiolene ligands are unsaturated
bidentate ligand
wherein the two donor atoms are sulfur
. Dithiolenes are often referred to as "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin
cofactor bound to the Mo or W.
Metal dithiolenes have been studied since the 1960s when they were first popularized by G. N. Schrauzer, who prepared Ni(S2C2Ph2)2 by the reaction of nickel sulfide and diphenylacetylene
. The structural, spectroscopic, and electrochemical properties of many related complexes have been described.
("mnt"), (NC)2C2S22-, and ethylenedithiolene, H2C2S22-.
of the ligand
(and therefore the metal centre) cannot be easily defined. Such ligands are therefore referred to as non-innocent
. The substituents on the backbone of the dithiolene ligand, R and R', affect the properties of the resulting metal complex in the expected way. Long chains confer solubility in less polar solvents. Electron acceptors (e.g. CN, CO2Me) stabilize reduced and anionic complexes. Derivatives are known where the substituents are the same, symmetrical dithiolenes (R = R') are more common than unsymmetrical.
Due to the delocalized nature of dithiolenes, metal dithiolenes often exist in multiple oxidation states. In oxidized dithiolene complexes have relatively more 1,2-dithioketone character. In reduced complexes, the ligand assumes more ene-1,2-dithiolate character. These descriptions are evaluated by examination of differences in C-C and C-S bond distances.
These limiting structures do not represent a true description of the actual structure of the complex, the true structure lies somewhere between. Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidations state. This suggestion was generally accepted, and 'dithiolene' is now a universally accepted term.
, which forms a very stable Ni(II) complex:
This red-colored complex is often used as a standard for electrochemical studies.
For more electron-rich alkenedithiolates, the dianion is generated in situ, treated with the metal salt, and the product is often air-oxidized:
s, with P4S10
followed by hydrolysis and treatment of the ill-defined mixture with metal salts. This method is used to prepare Ni[S2C2Ar2]2 (Ar = aryl).
, the dithiete
s are occasionally used. Probably the most important dithiete is the distillable (CF3)2C2S2, prepared from the reaction of elemental sulfur
and hexafluoro-2-butyne
. This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes.
. More modern versions of this method entail the reaction of electrophilic acetylenes such as dimethyl acetylenedicarboxylate
with well defined polysulfido complexes.
3.png)
, and nonlinear optics
. Relatively few dithiolene complexes have been commercialized for use as dyes in laser applications.
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
bidentate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
wherein the two donor atoms are sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. Dithiolenes are often referred to as "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
cofactor bound to the Mo or W.
Metal dithiolenes have been studied since the 1960s when they were first popularized by G. N. Schrauzer, who prepared Ni(S2C2Ph2)2 by the reaction of nickel sulfide and diphenylacetylene
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of phenyl groups attached to both ends of an alkyne. It is a colorless crystalline material that is widely used as a building block in organic and as a ligand in organometallic chemistry.-Preparation:Several...
. The structural, spectroscopic, and electrochemical properties of many related complexes have been described.
Background and nomenclature
Early studies on dithiolene ligands, although not called by that name until the 1960s, focused on the quinoxaline-2,3-dithiolates and 3,4-toluenedithiolates, which form brightly colored precipitates with several metal centres. Such species were originally of interest in analytical chemistry. Dithiolenes lacking benzene backbones represented an important development of the area, especially maleonitrile-2,3-ditholateSodium maleonitriledithiolate
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C22. The name refers to the cis compound, formally derived from maleonitrile. The dianion is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate that serves as a non-innocent ligand for transition metals...
("mnt"), (NC)2C2S22-, and ethylenedithiolene, H2C2S22-.
Electronic structure
Because of the unusual redox and intense optical properties of dithiolenes, their electronic structure of dithiolene complexes has been the subject of intense study. Dithiolene ligands can exist in three oxidation states: the dianionic "ene-1,2-dithiolate", the neutral "1,2-dithioketone," and a monoanionic radical intermediate between these two. When the latter two are complexed to a metal centre, the oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of the ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
(and therefore the metal centre) cannot be easily defined. Such ligands are therefore referred to as non-innocent
Non-innocent ligand
In chemistry, a non-innocent ligand is a ligand in a metal complex where the oxidation state is unclear. Typically, complexes containing non-innocent ligands are redox active at mild potentials...
. The substituents on the backbone of the dithiolene ligand, R and R', affect the properties of the resulting metal complex in the expected way. Long chains confer solubility in less polar solvents. Electron acceptors (e.g. CN, CO2Me) stabilize reduced and anionic complexes. Derivatives are known where the substituents are the same, symmetrical dithiolenes (R = R') are more common than unsymmetrical.
Due to the delocalized nature of dithiolenes, metal dithiolenes often exist in multiple oxidation states. In oxidized dithiolene complexes have relatively more 1,2-dithioketone character. In reduced complexes, the ligand assumes more ene-1,2-dithiolate character. These descriptions are evaluated by examination of differences in C-C and C-S bond distances.
These limiting structures do not represent a true description of the actual structure of the complex, the true structure lies somewhere between. Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidations state. This suggestion was generally accepted, and 'dithiolene' is now a universally accepted term.
From alkenedithiolates
Most dithiolene complexes are prepared by reaction of alkali metal salts of 1,2-alkenedithiolates with metal halides. A common alkenedithiolate is mnt2-Sodium maleonitriledithiolate
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C22. The name refers to the cis compound, formally derived from maleonitrile. The dianion is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate that serves as a non-innocent ligand for transition metals...
, which forms a very stable Ni(II) complex:
- Ni2+ + 2 (NC)2C2S22- → Ni[S2C2(CN)2]22-
This red-colored complex is often used as a standard for electrochemical studies.
For more electron-rich alkenedithiolates, the dianion is generated in situ, treated with the metal salt, and the product is often air-oxidized:
- cis-H2C2(SCH2Ph)2 + 4 Na → cis-H2C2(SNa)2 + 2 NaCH2Ph
- NiCl2 + 2 cis-H2C2(SNa)2 → Na2[Ni(S2C2H2)2] + 2 NaCl
- [Ni(S2C2H2)2]2- → Ni(S2C2H2)2 + 2 e-
From acyloins
An early and still powerful method for the synthesis of dithiolenes entails the reaction of α-hydroxyketones, acyloinAcyloin
Acyloins are a class of organic compounds in organic chemistry sharing a common functional group consisting of a hydroxyl group placed on the α-position of a carbonyl group.- Nomenclature :Common types of ketols include:...
s, with P4S10
Phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
followed by hydrolysis and treatment of the ill-defined mixture with metal salts. This method is used to prepare Ni[S2C2Ar2]2 (Ar = aryl).
From dithietes
Although 1,2-dithiones are extremely rare and thus not useful precursors, their valence isomerValence isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.-Benzene:...
, the dithiete
Dithiete
Dithietes are unsaturated heterocyclic compounds that contain two sulfur atoms and two sp2-hybridized carbon centers. Having 6 π electrons, dithietes are aromatic. Two isomers are possible for this small class of organosulfur compounds, 1,2-dithietes, where the sulfur atoms are adjacent, and...
s are occasionally used. Probably the most important dithiete is the distillable (CF3)2C2S2, prepared from the reaction of elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and hexafluoro-2-butyne
Hexafluoro-2-butyne
Hexafluoro-2-butyne is the fluorocarbon with the formula CF3C≡CCF3. HFB, as it is known also, is a particularly electrophilic acetylene, hence a potent dienophile....
. This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes.
- Mo(CO)6 + 3 1,2-(CF3)2C2S2 → [(CF3)2C2S2]3Mo + 6 CO
- Ni(CO)4 + 2 1,2-(CF3)2C2S2 → [(CF3)2C2S2]2Ni + 4 CO
By reactions of metal sulfides with alkynes
Species of the type Ni[S2C2Ar2]2 were first prepared by reactions of nickel sulfides with diphenylacetyleneDiphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of phenyl groups attached to both ends of an alkyne. It is a colorless crystalline material that is widely used as a building block in organic and as a ligand in organometallic chemistry.-Preparation:Several...
. More modern versions of this method entail the reaction of electrophilic acetylenes such as dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
with well defined polysulfido complexes.
Structural characteristics
Dithiolene complexes can be found where the metal centre is coordinated by one, two, or three dithiolene ligands. The tris(dithiolene) complexes were the first examples of trigonal prismatic geometry in coordination chemistry. One example is Mo(S2C2Ph2)3. Similar structures have been observed for several other metals.3.png)
Applications
Applications of 1,2-dithiolene complexes have been disccussed in the context of conductivity, magnetismMagnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
, and nonlinear optics
Nonlinear optics
Nonlinear optics is the branch of optics that describes the behavior of light in nonlinear media, that is, media in which the dielectric polarization P responds nonlinearly to the electric field E of the light...
. Relatively few dithiolene complexes have been commercialized for use as dyes in laser applications.

