
Dithiane
Encyclopedia
| ithiane |
|---|
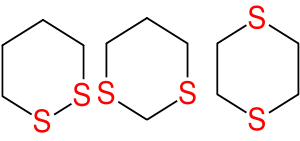 |
   |
A dithiane is a heterocyclic compound
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
composed of a cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
core structure wherein two methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
units are replaced by sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.
1,3-Dithianes
1,3-Dithianes are protecting groupProtecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
of some carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
-containing compounds due to their inertness to many conditions. They form by treatment of the carbonyl compound with 1,3-propanedithiol
1,3-Propanedithiol
1,3-Propanedithiol is the chemical compound with the formula HSCH2CH2CH2SH. This dithiol is a useful reagent in organic synthesis. This liquid, which is readily available commercially, has an intense stench.-Use in organic synthesis:...
under conditions that remove water from the system. The protecting group can be removed with mercuric reagents, a process that exploits the high affinity of Hg(II) for thiolates. 1,3-Dithianes are also employed in umpolung reactions. Typically, in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, ketones and aldehydes are protected as their dioxolane
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula 2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane is an peroxide...
s instead of dithianes.
See also
http://www.tricity.wsu.edu/Chem542/Forposting/jo00822a019.pdf older article of historic interest for the oxidative hydrolysis of 1,3-dithianes.http://www.organic-chemistry.org/namedreactions/corey-seebach-reaction.shtm
http://www.organic-chemistry.org/protectivegroups/carbonyl/1,3-dithiolanes.htm

