
Dihydrodipicolinate synthase
Encyclopedia
In enzymology, a dihydrodipicolinate synthase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are L-aspartate 4-semialdehyde and pyruvate, whereas its two products
are (S)-2,3-dihydropyridine-2,6-dicarboxylate and H2O
.
This enzyme belongs to the family of lyase
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is L-aspartate-4-semialdehyde hydro-lyase [adding pyruvate and cyclizing; (S)-2,3-dihydropyridine-2,6-dicarboxylate-forming]. Other names in common use include dihydropicolinate synthetase (DHDPS), dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and, and cyclizing). This enzyme participates in lysine biosynthesis.
Dihydropicolinate synthase is the key enzyme
in lysine
biosynthesis
via the diaminopimelate pathway of prokaryotes, some phycomycetes
and higher plants. The enzyme catalyses
the condensation of L-aspartate-beta- semialdehyde and pyruvate to dihydropicolinic acid via a ping-pong mechanism in which pyruvate binds to the enzyme by forming a Schiff base
with a lysine
residue. Three other protein
s are structurally related to DHDPS and probably also act via a similar catalytic mechanism. These are Escherichia coli
N-acetylneuraminate lyase
(gene nanA), which catalyses
the condensation of N-acetyl-D-mannosamine and pyruvate to form N-acetylneuraminate; Rhizobium meliloti (Sinorhizobium meliloti) protein mosA, which is involved in the biosynthesis of the rhizopine 3-o-methyl-scyllo-inosamine; and E. coli hypothetical protein yjhH. The sequence
s of DHDPS from different sources are well-conserved. The structure takes the form of a homotetramer, in which 2 monomer
s are related by an approximate 2-fold symmetry
. Each monomer comprises 2 domains: an 8-fold alpha-/beta-barrel, and a C-terminal alpha-helical domain. The fold
resembles that of N-acetylneuraminate lyase. The active site
lysine is located in the barrel domain, and has access via 2 channels on the C-terminal side of the barrel.
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , , , , , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- L-aspartate 4-semialdehyde + pyruvate
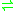 (S)-2,3-dihydropyridine-2,6-dicarboxylate + 2 H2O
(S)-2,3-dihydropyridine-2,6-dicarboxylate + 2 H2O
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are L-aspartate 4-semialdehyde and pyruvate, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are (S)-2,3-dihydropyridine-2,6-dicarboxylate and H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
.
This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is L-aspartate-4-semialdehyde hydro-lyase [adding pyruvate and cyclizing; (S)-2,3-dihydropyridine-2,6-dicarboxylate-forming]. Other names in common use include dihydropicolinate synthetase (DHDPS), dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and, and cyclizing). This enzyme participates in lysine biosynthesis.
Dihydropicolinate synthase is the key enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
in lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
via the diaminopimelate pathway of prokaryotes, some phycomycetes
Phycomycetes
Phycomycetes is an obsolete taxon for certain fungi with nonseptate hyphae.These fungi are currently classified under Zygomycota....
and higher plants. The enzyme catalyses
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the condensation of L-aspartate-beta- semialdehyde and pyruvate to dihydropicolinic acid via a ping-pong mechanism in which pyruvate binds to the enzyme by forming a Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
with a lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
residue. Three other protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s are structurally related to DHDPS and probably also act via a similar catalytic mechanism. These are Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
N-acetylneuraminate lyase
N-acetylneuraminate lyase
In enzymology, a N-acetylneuraminate lyase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, N-acetylneuraminate, and two products, N-acetyl-D-mannosamine and pyruvate....
(gene nanA), which catalyses
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the condensation of N-acetyl-D-mannosamine and pyruvate to form N-acetylneuraminate; Rhizobium meliloti (Sinorhizobium meliloti) protein mosA, which is involved in the biosynthesis of the rhizopine 3-o-methyl-scyllo-inosamine; and E. coli hypothetical protein yjhH. The sequence
Sequence (biology)
A sequence in biology is the one-dimensional ordering of monomers, covalently linked within in a biopolymer; it is also referred to as the primary structure of the biological macromolecule.-See also:* Protein sequence* DNA sequence...
s of DHDPS from different sources are well-conserved. The structure takes the form of a homotetramer, in which 2 monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s are related by an approximate 2-fold symmetry
Symmetry
Symmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
. Each monomer comprises 2 domains: an 8-fold alpha-/beta-barrel, and a C-terminal alpha-helical domain. The fold
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
resembles that of N-acetylneuraminate lyase. The active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
lysine is located in the barrel domain, and has access via 2 channels on the C-terminal side of the barrel.
Structural studies
As of late 2007, 16 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , , , , , , , , and .

