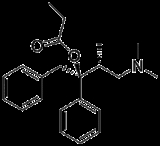
Dextropropoxyphene
Encyclopedia
Dextropropoxyphene, manufactured by Eli Lilly and Company
, is an analgesic
in the opioid
category. It is intended to treat mild pain and has, in addition, anti-tussive and local anesthetic effects. It has been taken off the market in Europe and the US due to concerns of fatal overdoses and arrhythmias. An estimated 10 million patients have used these products.
Dextropropoxyphene is sometimes combined with paracetamol
or acetylsalicylic acid. Trade-names include Darvocet-N and Di-Gesic Darvon with APAP for dextropropoxyphene and paracetamol and Darvon with ASA for dextropropoxyphene and aspirin
. The British Approved Name
(i.e. the generic name of the active ingredient) of the paracetamol/dextropropoxyphene preparation is co-proxamol (sold under a variety of brand names); however, it has been withdrawn since 2007, and is no longer available to new patients. The paracetamol combination(s) are known as Capadex or Di-Gesic in Australia
, Lentogesic in South Africa
, and Di-Antalvic in France
(unlike co-proxamol, which is an approved name, these are all brand names).
, is a weak opioid
, known to cause dependency among recreational users. Codeine is more commonly used; however, as codeine is, in essence, a prodrug that requires in vivo
metabolism to the more active opioid morphine for maximum efficacy, it is ineffective for some individuals with the "poor metabolizer" genotype of the liver cytochrome P450 enzyme CYP2D6
. It is in people with this low-function isoform of the CYP2D6 gene that dextropropoxyphene is particularly useful, as its metabolism does not require CYP2D6. It is also used for patients with digestive complaints as it is less liable to worsen their symptoms.
(RLS).
symptoms in people addicted to opioids. Being very weak in comparison to the opioids that are commonly abused, dextropropoxyphene can only act as a "partial" substitute. It does not have much effect on mental cravings; however it can be effective in alleviating physical withdrawal effects, such as muscle cramps.
to paracetamol or dextropropoxyphene, in alcoholics
; and in combination with amphetamine. Dextropropoxyphene is not intended for use in patients who are prone to suicide or addiction.
. It also acts as a potent, noncompetitive α3β4
neuronal nicotinic acetylcholine receptor
antagonist
, as well as weak serotonin reuptake inhibitor.
toxicity (from paracetamol poisoning) and dextropropoxyphene overdose.
Many users experience toxic effects from the paracetamol
(acetaminophen) in pursuit of the endlessly-increasing dose required for pain relief. They suffer acute liver toxicity
, which causes severe stomach pains, nausea
, and vomiting
(all of which are increased by light or stimulation of the sense of sight).
An overdose of dextropropoxyphene may lead to various systemic effects. Excessive opioid receptor stimulation is responsible for the CNS depression
, respiratory depression, miosis
, and gastrointestinal effects seen in propoxyphene poisoning. It may also account for mood
/thought
altering effects.
In addition, both propoxyphene and its metabolite norpropoxyphene
have local anesthetic effects at concentrations about 10 times those necessary for opioid effects. Norpropoxyphene is a more potent local anesthetic than propoxyphene, and they are both more potent than lidocaine
. Local anesthetic activity appears to be responsible for the arrhythmias and cardiovascular depression seen in propoxyphene poisoning.
Both propoxyphene and norpropoxyphene are potent blockers of cardiac membrane sodium channels and are more potent than lidocaine
, quinidine
, and procainamide
in this respect. As a result, propoxyphene and norpropoxyphene appear to have the characteristics of a Vaughn-Williams Class Ic antiarrhythmic.
These direct cardiac effects include decreased heart rate
(i.e. cardiovascular depression), decreased contractility
, and decreased electrical conductivity (i.e., increased PR, AH, HV, and QRS intervals). These effects appear to be due to their local anesthetic activity and are not reversed by naloxone
. Widening of the QRS complex appears to be a result of a quinidine-like effect of propoxyphene, and sodium bicarbonate
therapy appears to have a positive direct effect on the QRS dysrhythmia.
Seizure
s may result from either opioid or local anesthetic effects. Pulmonary edema
may result from direct pulmonary toxicity
, neurogenic/anoxic
effects, or cardiovascular depression.
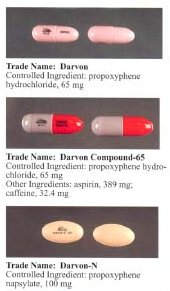
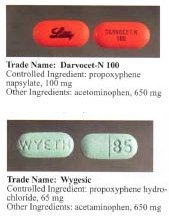
Before the FDA-directed recall, dextropropoxyphene HCl was available in the United States
as a prescription formulation with paracetamol
(acetaminophen) in ratio anywhere from 30 mg / 600 mg to 100 mg / 650 mg (or 100 mg / 325 mg in the case of Balacet), respectively. These are usually named "Darvocet." On the other hand, "Darvon" is a pure propoxyphene preparation that does not contain paracetamol.
In Australia, dextropropoxyphene is available on prescription, both as a combined product (32.5 mg dextropropoxyphene per 325 mg paracetamol
branded as either "Di-gesic", "Capadex", and "Paradex," it is also available in pure form (100 mg capsules) known as "Doloxene".
.
 Dextropropoxyphene is subject to some controversy: while many physician
Dextropropoxyphene is subject to some controversy: while many physician
s prescribe it for a wide range of mildly to moderately painful symptoms as well as for treatment of diarrhea
, many others refuse to prescribe it, citing limited effectiveness. In addition, the therapeutic index
of dextroproxyphene is relatively small.
Caution should be used when administering dextropropoxyphene, particularly with children and the elderly and with patients who may be pregnant or breast feeding; other reported problems include kidney, liver or respiratory disorders, and prolonged use. Attention should be paid to concomitant use with tranquilizers, antidepressants or excess alcohol.
Darvon, a dextropropoxyphene made by Eli Lilly
, which had been on the market for 25 years, came under heavy fire in 1978 by consumer groups that said it was associated with suicide
. Darvon was never withdrawn from the market, but Lilly has waged a sweeping, and largely successful, campaign among doctors, pharmacists and Darvon users to defend the drug as safe when it is used in proper doses and not mixed with alcohol. On November 19, 2010, the FDA banned all sale of Darvon and Darvocet.
, both pure dextropropoxyphene capsules (as napsylate, 100 mg), marketed as "Doloxene", and combination tablets and capsules (with paracetamol) all containing 32.5 mg dextropropoxyphene HCl with 325 mg paracetamol, which are currently available on prescription will be withdrawn from 1 March 2012.
requested the European Medicines Agency
(EMEA) to review the safety and effectiveness of dextropropoxyphene based medicines and on 25 June 2009 the EMEA recommended a gradual withdrawal throughout the European Union
. The EMEA's conclusion was based on evidence that dextropropoxyphene-containing medicines were weak painkillers, the combination of dextropropoxyphene and paracetamol was no more effective than paracetamol on its own, and that the difference between the dose needed for treatment and a harmful dose (the "therapeutic index
") was too small.
announced that Paradex and Capadex (forms of dextropropoxyphene) were being withdrawn from the marketplace due to health issues, and withdrawal in other countries.
physicians have earlier been discouraged by the medical products agency to prescribe dextropropoxyphene due to the risk of respiratory depression when taken with alcohol. Products with mixed active ingredients where taken off the market and only products with dextropropoxyphene where earlier supposed to be sold. Physicians have eariler been recommended to prescribe products with only dextropropoxyphene and not to patients with a history of drug abuse, depression or suicidal tendencies.
(MHRA) removed the licence for co-proxamol. From then onwards, in the UK co-proxamol is only available on a named patient basis, for long term chronic pain and only to those who have already been prescribed this medicine. Its withdrawal from the UK market is a result of concerns relating to its toxicity in overdose (even small overdose can be fatal), and dangerous reaction with alcohol. Recreational use in the UK is uncommon. Many patients have been prescribed alternative combinations of drugs as a replacement.
The MHRA's motivation for the withdrawal of co-proxamol was the reduction in suicides and a key part of its justification of its decision was based upon studies showing that co-proxamol was no more effective than paracetamol alone in pain management. Prescribing authorities such as the Royal College of General Practitioners unanimously recommended withdrawal, while patients who responded to the MHRA's request for information tended to want to continue treatment. Many doctors as well as patients believe that clinical experience shows co-proxamol is more effective than paracetamol alone.
The co-proxamol preparations available in the UK contained a sub-therapeutic dose of paracetamol, 325 mg per tablet. Patients were warned not to take more than eight tablets in one day, a total dose of 2600 mg paracetamol per day. This is in comparison to the 4000 mg daily limit on paracetamol alone, a significantly higher dose. Despite this reduced level, patients were still at a high risk of overdose: coproxamol was second only to tricyclic antidepressants as the most common prescription drugs used in overdose. Following the reduction in prescribing in 2005–2007, prior to its complete withdrawal, the number of deaths associated with the drug dropped significantly. Additionally, patients have not substituted other drugs as a method of overdose.
The decision to withdraw coproxamol has met with some controversy; it has been brought up in the House of Commons on two occasions, 13 July 2005 and on 17 January 2007. Patients have found alternatives to co-proxamol either too strong, too weak, or with intolerable side effects. During the House of Commons debates, it is quoted that originally some 1,700,000 patients in the UK were prescribed co-proxamol. Following the MHRA phased withdrawal this has eventually been reduced to 70,000. However, it appears this is the residual pool of patients who cannot find alternate analgesia to co-proxamol.
The MHRA safety net of prescribing co-proxamol after licence withdrawal from 31 December 2007, on a "Named Patient" basis where doctors agree there is a clinical need, has been rejected by most UK doctors because the MHRA wording that "responsibility will fall on the prescriber" is unacceptable to most doctors. Some patients intend to take the case to the European Court of Human Rights. However, the European Medicines Agency has recently backed the MHRA's decision, and recommended in June 2009 that propoxyphene preparations be withdrawn across the European Union.
in the U.S., stating:
Because of potential for side effects, this drug is on the list for High Risk Medications in the elderly.
On November 19, 2010, the FDA requested the cessation of all sale of Darvon and Darvocet from the US drug market due to heart arrhythmia in patients who took the drug. The drug Darvocet may also be involved in combined drug intoxication
because it may lead to confusion in patients and physicians. Many doctors are commonly switching to tramadol
, because it is generally considered safer.
's The Peaceful Pill Handbook
and Dr. Pieter Admiraal's Guide to a Human Self-Chosen Death. "With the withdrawal of the barbiturate
sleeping tablets from the medical prescribing list, propoxyphene has become the most common doctor-prescribed medication used by seriously ill people to end their lives." The slang name for the combination of propoxyphene and other drugs used for suicide is "Darvon cocktail".
Eli Lilly and Company
Eli Lilly and Company is a global pharmaceutical company. Eli Lilly's global headquarters is located in Indianapolis, Indiana, in the United States...
, is an analgesic
Analgesic
An analgesic is any member of the group of drugs used to relieve pain . The word analgesic derives from Greek an- and algos ....
in the opioid
Opioid
An opioid is a psychoactive chemical that works by binding to opioid receptors, which are found principally in the central and peripheral nervous system and the gastrointestinal tract...
category. It is intended to treat mild pain and has, in addition, anti-tussive and local anesthetic effects. It has been taken off the market in Europe and the US due to concerns of fatal overdoses and arrhythmias. An estimated 10 million patients have used these products.
Dextropropoxyphene is sometimes combined with paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
or acetylsalicylic acid. Trade-names include Darvocet-N and Di-Gesic Darvon with APAP for dextropropoxyphene and paracetamol and Darvon with ASA for dextropropoxyphene and aspirin
Aspirin
Aspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
. The British Approved Name
British Approved Name
A British Approved Name is the official non-proprietary or generic name given to a pharmaceutical substance, as defined in the British Pharmacopoeia...
(i.e. the generic name of the active ingredient) of the paracetamol/dextropropoxyphene preparation is co-proxamol (sold under a variety of brand names); however, it has been withdrawn since 2007, and is no longer available to new patients. The paracetamol combination(s) are known as Capadex or Di-Gesic in Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, Lentogesic in South Africa
South Africa
The Republic of South Africa is a country in southern Africa. Located at the southern tip of Africa, it is divided into nine provinces, with of coastline on the Atlantic and Indian oceans...
, and Di-Antalvic in France
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
(unlike co-proxamol, which is an approved name, these are all brand names).
Analgesia
Dextropropoxyphene, like codeineCodeine
Codeine or 3-methylmorphine is an opiate used for its analgesic, antitussive, and antidiarrheal properties...
, is a weak opioid
Opioid
An opioid is a psychoactive chemical that works by binding to opioid receptors, which are found principally in the central and peripheral nervous system and the gastrointestinal tract...
, known to cause dependency among recreational users. Codeine is more commonly used; however, as codeine is, in essence, a prodrug that requires in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
metabolism to the more active opioid morphine for maximum efficacy, it is ineffective for some individuals with the "poor metabolizer" genotype of the liver cytochrome P450 enzyme CYP2D6
CYP2D6
Cytochrome P450 2D6 , a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. Also, many substances are bioactivated by CYP2D6 to form their active compounds...
. It is in people with this low-function isoform of the CYP2D6 gene that dextropropoxyphene is particularly useful, as its metabolism does not require CYP2D6. It is also used for patients with digestive complaints as it is less liable to worsen their symptoms.
Restless legs syndrome (RLS)
Dextropropoxyphene has been found to be helpful in relieving the symptoms of restless legs syndromeRestless legs syndrome
Restless legs syndrome or Willis-Ekbom disease is a neurological disorder characterized by an irresistible urge to move one's body to stop uncomfortable or odd sensations. It most commonly affects the legs, but can affect the arms, torso, and even phantom limbs...
(RLS).
Opioid withdrawal
In pure form, dextropropoxyphene is commonly used to ease the withdrawalWithdrawal
Withdrawal can refer to any sort of separation, but is most commonly used to describe the group of symptoms that occurs upon the abrupt discontinuation/separation or a decrease in dosage of the intake of medications, recreational drugs, and alcohol...
symptoms in people addicted to opioids. Being very weak in comparison to the opioids that are commonly abused, dextropropoxyphene can only act as a "partial" substitute. It does not have much effect on mental cravings; however it can be effective in alleviating physical withdrawal effects, such as muscle cramps.
Contraindications
Dextropropoxyphene is contraindicated in patients allergicAllergy
An Allergy is a hypersensitivity disorder of the immune system. Allergic reactions occur when a person's immune system reacts to normally harmless substances in the environment. A substance that causes a reaction is called an allergen. These reactions are acquired, predictable, and rapid...
to paracetamol or dextropropoxyphene, in alcoholics
Alcoholism
Alcoholism is a broad term for problems with alcohol, and is generally used to mean compulsive and uncontrolled consumption of alcoholic beverages, usually to the detriment of the drinker's health, personal relationships, and social standing...
; and in combination with amphetamine. Dextropropoxyphene is not intended for use in patients who are prone to suicide or addiction.
Side effects
- Constipation
- Itching
- Drowsiness
- Sore throat
- Impaired alertness
- Confusion
- Serious or fatal heart rhythms


Pharmacology
Dextropropoxyphene acts as a mu-opioid receptor agonistAgonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
. It also acts as a potent, noncompetitive α3β4
Alpha-3 beta-4 nicotinic receptor
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the brain, where activation yields post- and presynaptic excitation....
neuronal nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptors, or nAChRs, are cholinergic receptors that form ligand-gated ion channels in the plasma membranes of certain neurons and on the postsynaptic side of the neuromuscular junction...
antagonist
Nicotinic antagonist
A nicotinic antagonist is a type of anticholinergic that inhibits the action at nicotinic acetylcholine receptors. These compounds are mainly used for peripheral muscle paralysis in surgery, but some centrally acting compounds such as bupropion, mecamylamine, and 18-methoxycoronaridine block...
, as well as weak serotonin reuptake inhibitor.
Toxicity
Overdose is commonly broken into two categories: liverLiver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
toxicity (from paracetamol poisoning) and dextropropoxyphene overdose.
Many users experience toxic effects from the paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
(acetaminophen) in pursuit of the endlessly-increasing dose required for pain relief. They suffer acute liver toxicity
Hepatotoxicity
Hepatotoxicity implies chemical-driven liver damage.The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure...
, which causes severe stomach pains, nausea
Nausea
Nausea , is a sensation of unease and discomfort in the upper stomach with an involuntary urge to vomit. It often, but not always, precedes vomiting...
, and vomiting
Vomiting
Vomiting is the forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose...
(all of which are increased by light or stimulation of the sense of sight).
An overdose of dextropropoxyphene may lead to various systemic effects. Excessive opioid receptor stimulation is responsible for the CNS depression
CNS depression
Central nervous system depression or CNS depression refers to physiological depression of the central nervous system that can result in decreased rate of breathing, decreased heart rate, and loss of consciousness possibly leading to coma or death...
, respiratory depression, miosis
Miosis
Miosis is the constriction of the pupil of the eye to two millimeters or less...
, and gastrointestinal effects seen in propoxyphene poisoning. It may also account for mood
Mood (psychology)
A mood is a relatively long lasting emotional state. Moods differ from emotions in that they are less specific, less intense, and less likely to be triggered by a particular stimulus or event....
/thought
Thought
"Thought" generally refers to any mental or intellectual activity involving an individual's subjective consciousness. It can refer either to the act of thinking or the resulting ideas or arrangements of ideas. Similar concepts include cognition, sentience, consciousness, and imagination...
altering effects.
In addition, both propoxyphene and its metabolite norpropoxyphene
Norpropoxyphene
Norpropoxyphene is a major metabolite of the opioid analgesic drug dextropropoxyphene, and is responsible for many of the side effects associated with use of this drug, especially the unusual toxicity seen during dextropropoxyphene overdose...
have local anesthetic effects at concentrations about 10 times those necessary for opioid effects. Norpropoxyphene is a more potent local anesthetic than propoxyphene, and they are both more potent than lidocaine
Lidocaine
Lidocaine , Xylocaine, or lignocaine is a common local anesthetic and antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations, injected as a dental anesthetic or as a local anesthetic for minor surgery.- History :Lidocaine, the first amino...
. Local anesthetic activity appears to be responsible for the arrhythmias and cardiovascular depression seen in propoxyphene poisoning.
Both propoxyphene and norpropoxyphene are potent blockers of cardiac membrane sodium channels and are more potent than lidocaine
Lidocaine
Lidocaine , Xylocaine, or lignocaine is a common local anesthetic and antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations, injected as a dental anesthetic or as a local anesthetic for minor surgery.- History :Lidocaine, the first amino...
, quinidine
Quinidine
Quinidine is a pharmaceutical agent that acts as a class I antiarrhythmic agent in the heart. It is a stereoisomer of quinine, originally derived from the bark of the cinchona tree.-Mechanism:...
, and procainamide
Procainamide
Procainamide INN is a pharmaceutical antiarrhythmic agent used for the medical treatment of cardiac arrhythmias, classified by the Vaughan Williams classification system as class Ia.-History:...
in this respect. As a result, propoxyphene and norpropoxyphene appear to have the characteristics of a Vaughn-Williams Class Ic antiarrhythmic.
These direct cardiac effects include decreased heart rate
Heart rate
Heart rate is the number of heartbeats per unit of time, typically expressed as beats per minute . Heart rate can vary as the body's need to absorb oxygen and excrete carbon dioxide changes, such as during exercise or sleep....
(i.e. cardiovascular depression), decreased contractility
Contractility
Myocardial contractility is the intrinsic ability of the heart to contract independent of preload and afterload. Changes in the ability to produce force during contraction result from different degrees of binding between myosin and actin filaments...
, and decreased electrical conductivity (i.e., increased PR, AH, HV, and QRS intervals). These effects appear to be due to their local anesthetic activity and are not reversed by naloxone
Naloxone
Naloxone is an opioid antagonist drug developed by Sankyo in the 1960s. Naloxone is a drug used to counter the effects of opiate overdose, for example heroin or morphine overdose. Naloxone is specifically used to counteract life-threatening depression of the central nervous system and respiratory...
. Widening of the QRS complex appears to be a result of a quinidine-like effect of propoxyphene, and sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
therapy appears to have a positive direct effect on the QRS dysrhythmia.
Seizure
Seizure
An epileptic seizure, occasionally referred to as a fit, is defined as a transient symptom of "abnormal excessive or synchronous neuronal activity in the brain". The outward effect can be as dramatic as a wild thrashing movement or as mild as a brief loss of awareness...
s may result from either opioid or local anesthetic effects. Pulmonary edema
Pulmonary edema
Pulmonary edema , or oedema , is fluid accumulation in the air spaces and parenchyma of the lungs. It leads to impaired gas exchange and may cause respiratory failure...
may result from direct pulmonary toxicity
Pulmonary toxicity
Pulmonary toxicity is the medical name for side effects on the lungs.Although most cases of pulmonary toxicity in medicine are due to side effects of medicinal drugs, many cases can be due to side effects of radiation...
, neurogenic/anoxic
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
effects, or cardiovascular depression.
Available forms
Propoxyphene was initially introduced as propoxyphene hydrochloride. Shortly before the patent on propoxyphene expired, propoxyphene napsylate form was introduced to the market. Napsylate salt is claimed to be less prone to abuse, because it is almost insoluble in water and therefore cannot be used for injection. Napsylate also gives lower peak blood level. Because of different molecular mass, a dose of 100 mg of propoxyphene napsylate is required to supply an amount of propoxyphene equivalent to that present in 65 mg propoxyphene hydrochloride.Before the FDA-directed recall, dextropropoxyphene HCl was available in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
as a prescription formulation with paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
(acetaminophen) in ratio anywhere from 30 mg / 600 mg to 100 mg / 650 mg (or 100 mg / 325 mg in the case of Balacet), respectively. These are usually named "Darvocet." On the other hand, "Darvon" is a pure propoxyphene preparation that does not contain paracetamol.
In Australia, dextropropoxyphene is available on prescription, both as a combined product (32.5 mg dextropropoxyphene per 325 mg paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
branded as either "Di-gesic", "Capadex", and "Paradex," it is also available in pure form (100 mg capsules) known as "Doloxene".
Drug testing
Detectable levels of propoxyphene/dextropropoxyphene may stay in a person's system for up to nine days after last dose and can be tested for specifically in non-standard urinalysis but may remain in the body longer in tiny amounts. Propoxyphene will not show up on standard opiate/opioid tests because it is not chemically related to opiates part of the OPI or OPI 2000 panels, which detect morphine and related compounds. It is most closely related to MethadoneMethadone
Methadone is a synthetic opioid, used medically as an analgesic and a maintenance anti-addictive for use in patients with opioid dependency. It was developed in Germany in 1937...
.
Usage controversy and regulation

Physician
A physician is a health care provider who practices the profession of medicine, which is concerned with promoting, maintaining or restoring human health through the study, diagnosis, and treatment of disease, injury and other physical and mental impairments...
s prescribe it for a wide range of mildly to moderately painful symptoms as well as for treatment of diarrhea
Diarrhea
Diarrhea , also spelled diarrhoea, is the condition of having three or more loose or liquid bowel movements per day. It is a common cause of death in developing countries and the second most common cause of infant deaths worldwide. The loss of fluids through diarrhea can cause dehydration and...
, many others refuse to prescribe it, citing limited effectiveness. In addition, the therapeutic index
Therapeutic index
The therapeutic index is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes death or toxicity ....
of dextroproxyphene is relatively small.
Caution should be used when administering dextropropoxyphene, particularly with children and the elderly and with patients who may be pregnant or breast feeding; other reported problems include kidney, liver or respiratory disorders, and prolonged use. Attention should be paid to concomitant use with tranquilizers, antidepressants or excess alcohol.
Darvon, a dextropropoxyphene made by Eli Lilly
Eli Lilly and Company
Eli Lilly and Company is a global pharmaceutical company. Eli Lilly's global headquarters is located in Indianapolis, Indiana, in the United States...
, which had been on the market for 25 years, came under heavy fire in 1978 by consumer groups that said it was associated with suicide
Suicide
Suicide is the act of intentionally causing one's own death. Suicide is often committed out of despair or attributed to some underlying mental disorder, such as depression, bipolar disorder, schizophrenia, alcoholism, or drug abuse...
. Darvon was never withdrawn from the market, but Lilly has waged a sweeping, and largely successful, campaign among doctors, pharmacists and Darvon users to defend the drug as safe when it is used in proper doses and not mixed with alcohol. On November 19, 2010, the FDA banned all sale of Darvon and Darvocet.
Australia
In AustraliaAustralia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, both pure dextropropoxyphene capsules (as napsylate, 100 mg), marketed as "Doloxene", and combination tablets and capsules (with paracetamol) all containing 32.5 mg dextropropoxyphene HCl with 325 mg paracetamol, which are currently available on prescription will be withdrawn from 1 March 2012.
European Union
In November 2007, the European CommissionEuropean Commission
The European Commission is the executive body of the European Union. The body is responsible for proposing legislation, implementing decisions, upholding the Union's treaties and the general day-to-day running of the Union....
requested the European Medicines Agency
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
(EMEA) to review the safety and effectiveness of dextropropoxyphene based medicines and on 25 June 2009 the EMEA recommended a gradual withdrawal throughout the European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
. The EMEA's conclusion was based on evidence that dextropropoxyphene-containing medicines were weak painkillers, the combination of dextropropoxyphene and paracetamol was no more effective than paracetamol on its own, and that the difference between the dose needed for treatment and a harmful dose (the "therapeutic index
Therapeutic index
The therapeutic index is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes death or toxicity ....
") was too small.
New Zealand
In February 2010, MedsafeMedsafe
Medsafe is the medical regulatory body run by the New Zealand Ministry of Health, administering the Medicines Act 1981 and Medicines Regulations 1984. Medsafe employs approximately 60 staff members in two offices...
announced that Paradex and Capadex (forms of dextropropoxyphene) were being withdrawn from the marketplace due to health issues, and withdrawal in other countries.
Sweden
In SwedenSweden
Sweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
physicians have earlier been discouraged by the medical products agency to prescribe dextropropoxyphene due to the risk of respiratory depression when taken with alcohol. Products with mixed active ingredients where taken off the market and only products with dextropropoxyphene where earlier supposed to be sold. Physicians have eariler been recommended to prescribe products with only dextropropoxyphene and not to patients with a history of drug abuse, depression or suicidal tendencies.
- As of march 2011 all products containing the substance are withdrawn because of safety issues after EU directive.′′
- It was discussed at the time that people who abuse alcohol and other substances and take combination dextropoxyphene/acetaminophen (paracetamol) may need to take many combination tablets to reach euphoria. This is because the amount of dextropropoxyphene per tablet is relatively low (30–40 mg). The ingested paracetamol - the other component - may then reach liver toxic levels. In the case of alcoholics, who often already have damaged livers, even a relatively small overdose with paracetamol may produce hepatotoxicityHepatotoxicityHepatotoxicity implies chemical-driven liver damage.The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure...
. The toxicity of the combination of overdosed dextropoxyphene with its CNS/respiratory depression and paracetamol induced liver damage can be a recipe for disaster or death. This is also true for any person with an already damaged or weakened liver by disease or otherwise.
United Kingdom
In the United Kingdom, preparations containing only dextropropoxyphene were discontinued in 2004. In 2007, the Medicines and Healthcare products Regulatory AgencyMedicines and Healthcare products Regulatory Agency
The Medicines and Healthcare products Regulatory Agency is the UK government agency which is responsible for ensuring that medicines and medical devices work and are acceptably safe....
(MHRA) removed the licence for co-proxamol. From then onwards, in the UK co-proxamol is only available on a named patient basis, for long term chronic pain and only to those who have already been prescribed this medicine. Its withdrawal from the UK market is a result of concerns relating to its toxicity in overdose (even small overdose can be fatal), and dangerous reaction with alcohol. Recreational use in the UK is uncommon. Many patients have been prescribed alternative combinations of drugs as a replacement.
The MHRA's motivation for the withdrawal of co-proxamol was the reduction in suicides and a key part of its justification of its decision was based upon studies showing that co-proxamol was no more effective than paracetamol alone in pain management. Prescribing authorities such as the Royal College of General Practitioners unanimously recommended withdrawal, while patients who responded to the MHRA's request for information tended to want to continue treatment. Many doctors as well as patients believe that clinical experience shows co-proxamol is more effective than paracetamol alone.
The co-proxamol preparations available in the UK contained a sub-therapeutic dose of paracetamol, 325 mg per tablet. Patients were warned not to take more than eight tablets in one day, a total dose of 2600 mg paracetamol per day. This is in comparison to the 4000 mg daily limit on paracetamol alone, a significantly higher dose. Despite this reduced level, patients were still at a high risk of overdose: coproxamol was second only to tricyclic antidepressants as the most common prescription drugs used in overdose. Following the reduction in prescribing in 2005–2007, prior to its complete withdrawal, the number of deaths associated with the drug dropped significantly. Additionally, patients have not substituted other drugs as a method of overdose.
The decision to withdraw coproxamol has met with some controversy; it has been brought up in the House of Commons on two occasions, 13 July 2005 and on 17 January 2007. Patients have found alternatives to co-proxamol either too strong, too weak, or with intolerable side effects. During the House of Commons debates, it is quoted that originally some 1,700,000 patients in the UK were prescribed co-proxamol. Following the MHRA phased withdrawal this has eventually been reduced to 70,000. However, it appears this is the residual pool of patients who cannot find alternate analgesia to co-proxamol.
The MHRA safety net of prescribing co-proxamol after licence withdrawal from 31 December 2007, on a "Named Patient" basis where doctors agree there is a clinical need, has been rejected by most UK doctors because the MHRA wording that "responsibility will fall on the prescriber" is unacceptable to most doctors. Some patients intend to take the case to the European Court of Human Rights. However, the European Medicines Agency has recently backed the MHRA's decision, and recommended in June 2009 that propoxyphene preparations be withdrawn across the European Union.
United States
In January 2009, an FDA advisory committee voted 14 to 12 against the continued marketing of propoxyphene products, based on its weak pain-killing abilities, addictiveness, association with drug deaths and possible heart problems, including arrhythmia. A subsequent re-evaluation resulted in a July 2009 recommendation to strengthen the boxed warning for propoxyphene to reflect the risk of overdose. Dextropropoxyphene currently carries a black box warningBlack box warning
In the United States, a black box warning is a type of warning that appears on the package insert for prescription drugs that may cause serious adverse effects...
in the U.S., stating:
Propoxyphene should be used with extreme caution, if at all, in patients who have a history of substance/drug/alcohol abuse, depression with suicidal tendency, or who already take medications that cause drowsiness (e.g., antidepressants, muscle relaxants, pain relievers, sedatives, tranquilizers). Fatalities have occurred in such patients when propoxyphene was misused.
Because of potential for side effects, this drug is on the list for High Risk Medications in the elderly.
On November 19, 2010, the FDA requested the cessation of all sale of Darvon and Darvocet from the US drug market due to heart arrhythmia in patients who took the drug. The drug Darvocet may also be involved in combined drug intoxication
Combined drug intoxication
Combined drug intoxication , also known as multiple drug intake or lethal polydrug/polypharmacy intoxication, is an unnatural cause of human death...
because it may lead to confusion in patients and physicians. Many doctors are commonly switching to tramadol
Tramadol
Tramadol hydrochloride is a centrally acting synthetic opioid analgesic used in treating moderate pain. The drug has a wide range of applications, including treatment for restless legs syndrome and fibromyalgia...
, because it is generally considered safer.
Canada
On December 1, 2010, Health Canada and Paladin Labs Inc. announced the voluntary recall and withdrawal of Darvon-N from the Canadian market and the discontinuation of sale of Darvon-N.Use by right to die societies
High toxicity and relatively easy availability made propoxyphene drug of choice for right to die societies. Propoxyphene is listed in Dr. Philip NitschkePhilip Nitschke
Dr. Philip Nitschke is an Australian medical doctor, humanist, author and founder and director of the pro-euthanasia group Exit International. He campaigned successfully to have a legal euthanasia law passed in Australia's Northern Territory and assisted four people in ending their lives before...
's The Peaceful Pill Handbook
The Peaceful Pill Handbook
The Peaceful Pill Handbook is a controversial book giving instructions on how to perform euthanasia. It was originally published in the U.S. in 2007 and was written by the Australian doctors Philip Nitschke and Fiona Stewart....
and Dr. Pieter Admiraal's Guide to a Human Self-Chosen Death. "With the withdrawal of the barbiturate
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
sleeping tablets from the medical prescribing list, propoxyphene has become the most common doctor-prescribed medication used by seriously ill people to end their lives." The slang name for the combination of propoxyphene and other drugs used for suicide is "Darvon cocktail".

