
D-cysteine desulfhydrase
Encyclopedia
In enzymology, a D-cysteine desulfhydrase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are D-cysteine and H2O
, whereas its 3 products
are sulfide
, NH3
, and pyruvate.
This enzyme belongs to the family of lyase
s, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is D-cysteine sulfide-lyase (deaminating; pyruvate-forming). Other names in common use include D-cysteine lyase, and D-cysteine sulfide-lyase (deaminating). This enzyme participates in cysteine metabolism
.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- D-cysteine + H2O
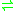 sulfide + NH3 + pyruvate
sulfide + NH3 + pyruvate
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are D-cysteine and H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
, NH3
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, and pyruvate.
This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is D-cysteine sulfide-lyase (deaminating; pyruvate-forming). Other names in common use include D-cysteine lyase, and D-cysteine sulfide-lyase (deaminating). This enzyme participates in cysteine metabolism
Cysteine metabolism
Cysteine metabolism refers to the biological pathways that consume or create cysteine. The pathways of different amino acids and other metabolites interweave and overlap to creating complex systems.-Human cysteine metabolism:...
.

