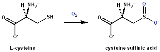
Cysteine dioxygenase
Encyclopedia
Cysteine dioxygenase) is a mammalian non-heme
iron enzyme
that catalyzes the conversion of L-cysteine
to cysteine sulfinic acid
(cysteine sulfinate) by incorporation of dioxygen.
 Cysteine sulfinic acid lies at a branch-point in cysteine catabolism, where it can follow two pathways resulting in the formation of taurine or sulfate
Cysteine sulfinic acid lies at a branch-point in cysteine catabolism, where it can follow two pathways resulting in the formation of taurine or sulfate
. The cysteine sulfinic acid-dependent pathway of taurine metabolism follows the synthesis of hypotaurine
(2-aminoethane sulfinate), which is subsequently oxidized to taurine. Also, cysteine sulfinate can undergo transamination to form β-sulfinylpyruvate, decomposing to form pyruvate and sulfite
.
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
iron enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes the conversion of L-cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
to cysteine sulfinic acid
Cysteine sulfinic acid
Cysteine sulfinic acid is an intermediate in cysteine metabolism.It is formed by cysteine dioxygenase....
(cysteine sulfinate) by incorporation of dioxygen.

Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
. The cysteine sulfinic acid-dependent pathway of taurine metabolism follows the synthesis of hypotaurine
Hypotaurine
Hypotaurine is a sulfinic acid that is an intermediate in the biosynthesis of taurine. Like taurine, it also acts as an endogenous neurotransmitter via action on the glycine receptors.- References :...
(2-aminoethane sulfinate), which is subsequently oxidized to taurine. Also, cysteine sulfinate can undergo transamination to form β-sulfinylpyruvate, decomposing to form pyruvate and sulfite
Sulfite
Sulfites are compounds that contain the sulfite ion SO. The sulfite ion is the conjugate base of bisulfite. Although the acid itself is elusive, its salts are widely used.-Structure:...
.

