Curtin-Hammett principle
Encyclopedia
The Curtin–Hammett principle is a principle in chemical kinetics
proposed by David Yarrow Curtin and Louis Plack Hammett
. It states that, for a reaction that has a pair of reactive intermediate
s or reactants that interconvert rapidly (as is usually the case for conformers), each going irreversibly to a different product, the product
ratio will depend only on the difference in the free energy
of the transition state
going to each product, and will not necessarily reflect the equilibrium distribution of the two intermediates.
s, diastereomer
s, or constitutional isomers. Product formation must be irreversible, and the different products must be unable to interconvert.
For example, given species A and B that equilibrate rapidly while A turns irreversibly into C, and B turns irreversibly into D:

K is the equilibrium constant between A and B, and k1 and k2 are the rate constants for the formation of C and D, respectively. When the rate of interconversion between A and B is much faster than either k1 or k2, then the Curtin–Hammett principle tells us that the C:D product ratio is not equal to the A:B reactant ratio, but is instead determined by the relative energy of the transition states.
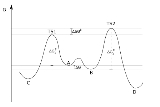
The reaction coordinate
free energy profile can be represented by the following scheme:
The ratio of products depends on the value labeled ΔΔG‡ in the figure: C will be the major product, because the energy of TS1 is lower than the energy of TS2. The commonly made assertion that the product distribution does not in any way reflect the relative free energies of substrates A and B is incorrect, however. As shown in the derivation below, the product ratio can be expressed solely as a function of ΔΔG‡, or as a function of K, k1, and k2.
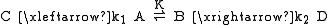
The rate of formation for compound C from A is given as
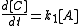
and that of D from B as:
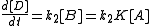
with Kc the equilibrium constant. The ratio of the rates is then:
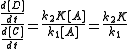
Because equilibration is fast compared to product formation, the ratio [B]/[A] remains unchanged throughout the reaction and K is constant. The product ratio can therefore be written as:
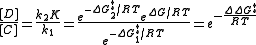
of tropanes with methyl iodide is a classic example of a Curtin-Hammett scenario in which a major product can arise from a less stable conformation. Here, the less stable conformer reacts via a more stable transition state to form the major product. Therefore, the ground state conformational distribution does not reflect the product distribution.
and enantioselective lithiation.
ic conformers and irreversible hydrogenation place the reaction under Curtin-Hammett control. The use of a chiral catalyst results in a higher-energy and a lower-energy transition state
for hydrogenation of the two enantiomers. The transformation occurs via the lower-energy transition state to form the product as a single enantiomer.
Consistent with the Curtin–Hammett principle, the ratio of products depends on the absolute energetic barrier of the irreversible step of the reaction, and does not reflect the equilibrium distribution of substrate conformers. The relative free energy profile of one example of the Noyori asymmetric hydrogenation is shown below:
product being formed in its absence.
Equilibration between the two alkyllithium complexes was demonstrated by the observation that enantioselectivity remained constant over the course of the reaction. Were the two reactant complexes not rapidly interconverting, enantioselectivity would erode over time as the faster-reacting conformer was depleted.
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
proposed by David Yarrow Curtin and Louis Plack Hammett
Louis Plack Hammett
Louis Plack Hammett was an American physical chemist. He is known for the Hammett equation, which relates reaction rates to equilibrium constants for certain classes of organic reactions involving substituted aromatic compounds...
. It states that, for a reaction that has a pair of reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s or reactants that interconvert rapidly (as is usually the case for conformers), each going irreversibly to a different product, the product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
ratio will depend only on the difference in the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
going to each product, and will not necessarily reflect the equilibrium distribution of the two intermediates.
Definition
The Curtin–Hammett principle applies to systems in which different products are formed from two substrates in equilibrium with one another. The rapidly interconverting reactants can be enantiomerEnantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s, diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s, or constitutional isomers. Product formation must be irreversible, and the different products must be unable to interconvert.
For example, given species A and B that equilibrate rapidly while A turns irreversibly into C, and B turns irreversibly into D:

K is the equilibrium constant between A and B, and k1 and k2 are the rate constants for the formation of C and D, respectively. When the rate of interconversion between A and B is much faster than either k1 or k2, then the Curtin–Hammett principle tells us that the C:D product ratio is not equal to the A:B reactant ratio, but is instead determined by the relative energy of the transition states.
The reaction coordinate
Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities....
free energy profile can be represented by the following scheme:
The ratio of products depends on the value labeled ΔΔG‡ in the figure: C will be the major product, because the energy of TS1 is lower than the energy of TS2. The commonly made assertion that the product distribution does not in any way reflect the relative free energies of substrates A and B is incorrect, however. As shown in the derivation below, the product ratio can be expressed solely as a function of ΔΔG‡, or as a function of K, k1, and k2.
Derivation
A generic reaction under Curtin-Hammett can be described by the following parameters: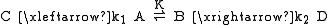
The rate of formation for compound C from A is given as
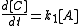
and that of D from B as:
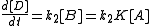
with Kc the equilibrium constant. The ratio of the rates is then:
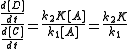
Because equilibration is fast compared to product formation, the ratio [B]/[A] remains unchanged throughout the reaction and K is constant. The product ratio can therefore be written as:
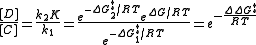
Classes of reactions under Curtin-Hammett control
Three main classes of reactions can be explained by the Curtin–Hammett principle: either the more or less stable conformer may react more quickly, or they may both react at the same rate.Case I: More stable conformer reacts more quickly
One category of reactions under Curtin-Hammett control includes transformations in which the more stable conformer reacts more quickly. This occurs when the transition state from the major intermediate to its respective product is lower in energy than the transition state from the minor intermediate to the other possible product. The major product is then derived from the major conformer, and the product distribution does not mirror the equilibrium conformer distribution.Example: Piperidine Oxidation
An example of a Curtin-Hammett scenario in which the more stable conformational isomer reacts more quickly is observed during the oxidation of piperidines. In the case of N-methyl piperidine, inversion at nitrogen between diasteriomeric conformers is much faster than the rate of amine oxidation. The conformation which places the methyl group in the equatorial position is 3.16 kcal/mol more stable than the equatorial conformation. The product ratio of 95:5 indicates that the more stable conformer leads to the major product.Case II: Less stable conformer reacts more quickly
A second category of reactions under Curtin-Hammett control includes those in which the less stable conformer reacts more quickly. In this case, despite an energetic preference for the less reactive species, the major product is derived from the higher-energy species. An important implication is that the product of a reaction can be derived from a conformer that is at sufficiently low concentration as to be unobservable in the ground state.Example: Tropane Alkylation
The alkylationAlkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of tropanes with methyl iodide is a classic example of a Curtin-Hammett scenario in which a major product can arise from a less stable conformation. Here, the less stable conformer reacts via a more stable transition state to form the major product. Therefore, the ground state conformational distribution does not reflect the product distribution.
Case III: Both conformers react at the same rate
It is hypothetically possible that two different conformers in equilibrium could react through transition states that are equal in energy. In this case, both conformers would react at the same rate. Eliel has proposed that the hypothetical reaction of cyclohexyl iodide with radiolabeled iodide would result in a completely symmetric transition state. Because both the equatorial and axial-substituted conformers would react through the same transition state, ΔΔG‡ would equal zero. By the Curtin–Hammett principle, the distribution of products should then be 50% axial substituted and 50% equatorial substituted. However, equilibration of the products precludes observation of this phenomenon.Application to stereoselective reactions
The Curtin–Hammett principle is used to explain the selectivity ratios for some stereoselective reactions.Application to dynamic kinetic resolution
The Curtin–Hammett principle can explain the observed dynamics in transformations employing dynamic kinetic resolution, such as the Noyori asymmetric hydrogenationNoyori asymmetric hydrogenation
The Noyori asymmetric hydrogenation is a chemical reaction described as an asymmetric reduction of β-keto-esters.Both enantiomers of BINAP are commercially available and widely used...
and enantioselective lithiation.
Noyori asymmetric hydrogenation
Rapid equilibration between enantiomerEnantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
ic conformers and irreversible hydrogenation place the reaction under Curtin-Hammett control. The use of a chiral catalyst results in a higher-energy and a lower-energy transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
for hydrogenation of the two enantiomers. The transformation occurs via the lower-energy transition state to form the product as a single enantiomer.
Consistent with the Curtin–Hammett principle, the ratio of products depends on the absolute energetic barrier of the irreversible step of the reaction, and does not reflect the equilibrium distribution of substrate conformers. The relative free energy profile of one example of the Noyori asymmetric hydrogenation is shown below:
Enantioselective lithiation
Dynamic kinetic resolution under Curtin-Hammett conditions has also been applied to enantioselective lithiation reactions. In the reaction below, it was observed that product enantioselectivities were independent of the chirality of the starting material. The use of (-)-sparteine is essential to enantioselectivity, with racemicRacemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
product being formed in its absence.
Equilibration between the two alkyllithium complexes was demonstrated by the observation that enantioselectivity remained constant over the course of the reaction. Were the two reactant complexes not rapidly interconverting, enantioselectivity would erode over time as the faster-reacting conformer was depleted.

