
Charge ordering
Encyclopedia
Charge ordering is a (first- or second-order) phase transition
occurring mostly in strongly correlated materials such as transition metal oxides
or organic conductors. Due to the strong interaction, the charge
is localized on different sites leading to a disproportionation and an ordered superlattice
. It appears in different patterns ranging from vertical to horizontal stripes to a checkerboard–like pattern, and it is not limited to the two-dimensional case. The charge order transition is accompanied by symmetry breaking
and ferroelectricity
. It is often brought in context with superconductivity
and colossal magnetoresistance
.
This long range order phenomena was first discovered in the magnetite Fe3O4 by Verwey in 1939.
He observed an increase of the electrical resistivity by two orders of magnitude at TCO=120K, suggesting a phase transition which is now well-known as the Verwey transition. He was the first bringing up the idea of an ordering process in this context.
delivers a good description of the charge order transition with the on-site and nearest neighbor Coulomb repulsion U and V. It emerged that V is a crucial parameter and important for developing the charge order state. Further model calculations try to take the temperature and an interchain interaction into account.
The extended Hubbard model for a single chain including inter-site and on-site interaction V and U as well as the parameter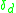 for a small dimerization which can be typically found in the (TMTTF)2X compounds is presented as follows:
for a small dimerization which can be typically found in the (TMTTF)2X compounds is presented as follows:
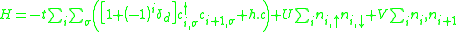
where t describes the transfer integral or the kinetic energy of the electron and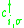 and
and 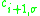 are the creation respectively the annihilation operator for an electron with the spin
are the creation respectively the annihilation operator for an electron with the spin 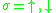 at the
at the 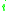 th or
th or  th site.
th site. 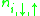 denotes the density operator. For non-dimerized systems,
denotes the density operator. For non-dimerized systems, 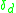 can be set to zero Normally, the on-site Coulomb repulsion U stays unchanged only t and V can vary with pressure.
can be set to zero Normally, the on-site Coulomb repulsion U stays unchanged only t and V can vary with pressure.
and acceptor
molecules building separated planar sheets or columns. The energy difference in the ionization
energy acceptor and the electron affinity
of the donor leads to a charge transfer and consequently to free carriers whose number is normally fixed. The carriers are delocalized throughout the crystal due to the overlap of the molecular orbitals being also reasonable for the high anisotropic conductivity. That is why it will be distinct between different dimensional organic conductors. They possess a huge variety of ground states, for instance, charge ordering, spin-Peierls, spin-density wave, antiferromagnetic
state, superconductivity
, charge-density wave to name only some of them.
-Fabre salts family, (TMTTF)2X and (TMTSF)2X, where in the latter one sulfur
is substituted by selenium
leading to a more metallic behavior over a wide temperature range and exhibiting no charge order. While the TMTTF compounds depending on the counterions X show the conductivity of a semiconductor at room temperature and are expected to be more one-dimensional than (TMTSF)2X.
The transition temperature TCO for the TMTTF subfamily was registered over two order of magnitudes for the centrosymmetric anions X = Br, PF6, AsF6, SbF6 and the non-centrosymmetric anions X= BF4 and ReO4.
In the middle of the eighties, a new "structureless transition" was discovered by Coulon et al. conducting transport and thermopower measurements. They observed a suddenly rise of the resistivity and the thermopower at TCO while x-ray measurements showed no evidence for a change in the crystal symmetry or a formation of a superstructure. The transition was later confirmed by 13C-NMR and dielectric measurements.
Different measurements under pressure reveal a decrease of the transition temperature TCO by increasing the pressure. According to the phase diagram of that family, an increasing pressure applied to the TMTTF compounds can be understood as a shift from the semiconducting state (at room temperature) to a higher dimensional and metallic state as you can find for TMTSF compounds without a charge order state.
with a high TC. The key to reach that aim was to increase the orbital overlap in two dimension. With the BEDT-TTF and its huge π-electron system, a new family of quasi two-dimensional organic conductors were created exhibiting also a great variety of the phase diagram and crystal structure arrangements.
At the turn of the 20th century, first NMR
measurements on the θ-(BEDT-TTF)2RbZn(SCN)4 compound uncovered the known metal to insulator transition at TCO= 195 K as an charge order transition.
Fe3O4 being a mixed-valence oxide where the iron atoms have a statistical distribution of Fe3+ and Fe2+ above the transition temperature. Below 122 K, the combination of 2+ and 3+ species arrange themselves in a regular pattern, whereas above that transition temperature (also referred to as the Verwey temperature in this case) the thermal energy is large enough to destroy the order.
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
occurring mostly in strongly correlated materials such as transition metal oxides
Transition metal oxides
Transition metal oxides comprise a class of materials that contain transition elements and oxygen. They include insulators as well as metals. Often the same material may display both types of transport properties, hence a Metal-Insulator transition, obtained by varying either temperature or...
or organic conductors. Due to the strong interaction, the charge
Charge (physics)
In physics, a charge may refer to one of many different quantities, such as the electric charge in electromagnetism or the color charge in quantum chromodynamics. Charges are associated with conserved quantum numbers.-Formal definition:...
is localized on different sites leading to a disproportionation and an ordered superlattice
Superlattice
Superlattice is a periodic structure of layers of two materials. Typically, the thickness of one layer is several nanometers.- Discovery :Superlattices were discovered early in the 20th century through their special X-ray diffraction patterns....
. It appears in different patterns ranging from vertical to horizontal stripes to a checkerboard–like pattern, and it is not limited to the two-dimensional case. The charge order transition is accompanied by symmetry breaking
Symmetry breaking
Symmetry breaking in physics describes a phenomenon where small fluctuations acting on a system which is crossing a critical point decide the system's fate, by determining which branch of a bifurcation is taken. To an outside observer unaware of the fluctuations , the choice will appear arbitrary...
and ferroelectricity
Ferroelectricity
Ferroelectricity is a property of certain materials which possess a spontaneous electric polarization that can be reversed by the application of an external electric field. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was...
. It is often brought in context with superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
and colossal magnetoresistance
Colossal magnetoresistance
Colossal magnetoresistance is a property of some materials, mostly manganese-based perovskite oxides, that enables them to dramatically change their electrical resistance in the presence of a magnetic field...
.
This long range order phenomena was first discovered in the magnetite Fe3O4 by Verwey in 1939.
He observed an increase of the electrical resistivity by two orders of magnitude at TCO=120K, suggesting a phase transition which is now well-known as the Verwey transition. He was the first bringing up the idea of an ordering process in this context.
Theoretical Description
The extended one-dimensional Hubbard modelHubbard model
The Hubbard model is an approximate model used, especially in solid state physics, to describe the transition between conducting and insulating systems...
delivers a good description of the charge order transition with the on-site and nearest neighbor Coulomb repulsion U and V. It emerged that V is a crucial parameter and important for developing the charge order state. Further model calculations try to take the temperature and an interchain interaction into account.
The extended Hubbard model for a single chain including inter-site and on-site interaction V and U as well as the parameter
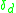 for a small dimerization which can be typically found in the (TMTTF)2X compounds is presented as follows:
for a small dimerization which can be typically found in the (TMTTF)2X compounds is presented as follows: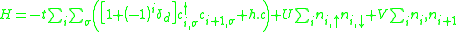
where t describes the transfer integral or the kinetic energy of the electron and
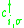 and
and 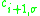 are the creation respectively the annihilation operator for an electron with the spin
are the creation respectively the annihilation operator for an electron with the spin 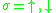 at the
at the 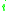 th or
th or  th site.
th site. 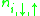 denotes the density operator. For non-dimerized systems,
denotes the density operator. For non-dimerized systems, 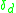 can be set to zero Normally, the on-site Coulomb repulsion U stays unchanged only t and V can vary with pressure.
can be set to zero Normally, the on-site Coulomb repulsion U stays unchanged only t and V can vary with pressure.Organic conductors
Organic conductors consist of donorDonor
A donor in general is a person who donates something voluntarily. Usually used to represent a form of pure altruism but sometimes used when the payment for a service is recognised by all parties as representing less than the value of the donation and that the motivation is altruistic...
and acceptor
Acceptor
Acceptor may refer to:* Acceptor , the addressee of a bill of exchange.* In the Indian Contract Act of 1872, the acceptor is the person to whom a proposal is made, and who has communicated his or her acceptance of the said proposal....
molecules building separated planar sheets or columns. The energy difference in the ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
energy acceptor and the electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
of the donor leads to a charge transfer and consequently to free carriers whose number is normally fixed. The carriers are delocalized throughout the crystal due to the overlap of the molecular orbitals being also reasonable for the high anisotropic conductivity. That is why it will be distinct between different dimensional organic conductors. They possess a huge variety of ground states, for instance, charge ordering, spin-Peierls, spin-density wave, antiferromagnetic
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usuallyrelated to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism...
state, superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
, charge-density wave to name only some of them.
Quasi One-dimensional Organic Conductors
The model system of one-dimensional conductors is the BechgaardBechgaard salt
A Bechgaard salt is any one of a number of organic charge-transfer complexes that exhibit superconductivity at low temperatures . They are named for chemist Klaus Bechgaard, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist...
-Fabre salts family, (TMTTF)2X and (TMTSF)2X, where in the latter one sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
is substituted by selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
leading to a more metallic behavior over a wide temperature range and exhibiting no charge order. While the TMTTF compounds depending on the counterions X show the conductivity of a semiconductor at room temperature and are expected to be more one-dimensional than (TMTSF)2X.
The transition temperature TCO for the TMTTF subfamily was registered over two order of magnitudes for the centrosymmetric anions X = Br, PF6, AsF6, SbF6 and the non-centrosymmetric anions X= BF4 and ReO4.
In the middle of the eighties, a new "structureless transition" was discovered by Coulon et al. conducting transport and thermopower measurements. They observed a suddenly rise of the resistivity and the thermopower at TCO while x-ray measurements showed no evidence for a change in the crystal symmetry or a formation of a superstructure. The transition was later confirmed by 13C-NMR and dielectric measurements.
Different measurements under pressure reveal a decrease of the transition temperature TCO by increasing the pressure. According to the phase diagram of that family, an increasing pressure applied to the TMTTF compounds can be understood as a shift from the semiconducting state (at room temperature) to a higher dimensional and metallic state as you can find for TMTSF compounds without a charge order state.
| Anion X | TCO (K) |
|---|---|
| (TMTTF)2Br | 28 |
| (TMTTF)2PF6 | 70 |
| (TMTTF)2AsF6 | 100.6 |
| (TMTTF)2SbF6 | 154 |
| (TMTTF)2BF4 | 83 |
| (TMTTF)2ReO4 | 227.5 |
| (DI-DCNQI)2Ag | 220 |
| TTM-TTPI3 | 120 |
Quasi Two-dimensional Organic Conductors
A dimensional crossover can be induced not only by applying pressure, but also be substituting the donor molecules by other ones. From a historical point of view, the main aim was to synthesize a organic superconductorOrganic superconductor
In physical chemistry and condensed matter physics, an organic superconductor is an organic compound which exhibits superconductivity at low temperatures...
with a high TC. The key to reach that aim was to increase the orbital overlap in two dimension. With the BEDT-TTF and its huge π-electron system, a new family of quasi two-dimensional organic conductors were created exhibiting also a great variety of the phase diagram and crystal structure arrangements.
At the turn of the 20th century, first NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
measurements on the θ-(BEDT-TTF)2RbZn(SCN)4 compound uncovered the known metal to insulator transition at TCO= 195 K as an charge order transition.
| Compound | TCO (K) |
|---|---|
| α-(BEDT-TTF)2I3 | 135 |
| θ-(BEDT-TTF)2TlCo(SCN)4 | 240 |
| θ-(BEDT-TTF)2TlZn(SCN)4 | 165 |
| θ-(BEDT-TTF)2RbZn(SCN)4 | 195 |
| θ-(BEDT-TTF)2RbCo(SCN)4 | 190 |
Transition metal oxides
The most prominent transition metal oxide revealing a CO transition is the magnetiteMagnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
Fe3O4 being a mixed-valence oxide where the iron atoms have a statistical distribution of Fe3+ and Fe2+ above the transition temperature. Below 122 K, the combination of 2+ and 3+ species arrange themselves in a regular pattern, whereas above that transition temperature (also referred to as the Verwey temperature in this case) the thermal energy is large enough to destroy the order.
| Compound | TCO (K) |
|---|---|
| Y0.5NiO3 | 582 |
| YBaCo2O5 | 220 |
| CaFeO3 | 290 |
| TbBaFe2O5 | 282 |
| Fe3O4 | 122 |
| Li0.5MnO2 | 290 |
| LaSrMn3O7 | 210 |
| Na0.25Mn3O6 | 176 |
| YBaMn2O6 | 498 |
| TbBaMn2O6 | 473 |
| PrCaMn2O6 | 230 |
| α'-NaV2O5 | 34 |
Detection of charge order
- NMRNMRNMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectroscopy is a powerful tool to measure the charge disproportionation. To apply this method to a certain system, it has to be doped with a nuclei, for instance 13C as it is the case for TMTTF compounds, being active for NMR. The local probe nuclei is very sensitive to the charge on the molecule observable in the Knight shiftKnight shiftThe Knight shift is a shift in the nuclear magnetic resonance frequency of a paramagneticsubstance first published in 1949 by the American physicist Walter David Knight.The Knight shift is due to the conduction electrons in metals...
K and the chemical shiftChemical shiftIn nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
D. The Knight shift K is proportional to the spin susceptibilitySusceptibility*In physics, the susceptibility of a material or substance describes its response to an applied field. There are many kinds of susceptibilities, for example:These two susceptibilities are particular examples of a linear response function;...
χSp on the molecule. The charge order or charge disproportionation appear as a splitting or broadening of the certain feature in the spectrum. - The X-ray diffractionX-ray crystallographyX-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
technique allows to determine the atomic position, but the extinction effect hinders to receive a high resolution spectrum. In the case of the organic conductors, the charge per molecule is measured by the change of the bond length of the C=C double bonds in the TTF molecule. A further problem arising by irradiating the organic conductors with x-rays is the destruction of the CO state. - In the organic molecules like TMTTF, TMTSF or BEDT-TFF, there are charge-sensitive modes changing their frequency depending on the local charge. Especially the C=C double bonds are quite sensitive to the charge. If a vibrational mode is infrared active or only visible in the RamanRaman spectroscopyRaman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
spectrum depends on its symmetry. In the case of BEDT-TTF, the most sensitive ones are the RamanRaman spectroscopyRaman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
active ν3 , ν2 and the infrared out of phase mode ν27. Their frequency is linearly associated to the charge per molecule giving the opportunity to determine the degree of disproportionation. - The charge order transition is also a metal to insulator transition being observable in transport measurements as a sharp rise in the resistivity. Transport measurements are therefore a good tool to get first evidences of a possible charge order transition.

