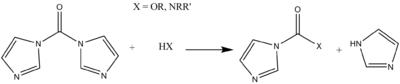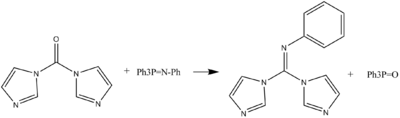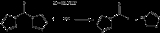
Carbonyldiimidazole
Encyclopedia
1,1'-Carbonyldiimidazole (CDI) is an organic compound
with the molecular formula (C3H3N2)2CO. It is a white crystalline solid
. It is often used for the coupling of amino acid
s for peptide
synthesis and as a reagent in organic synthesis
.
with four equivalents of imidazole
under anhydrous conditions. Removal of the side product, imidazolium chloride, and solvent results in the crystalline product in ~90% yield.
In this conversion, the imidazole serves both as the nucleophile and the base. An alternative precursor 1-(trimethylsilyl)imidazole requires more preparative effort with no corresponding advantages.
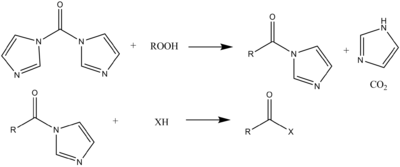
CDI hydrolyzes readily to give back imidazole:2CO + H2O → 2 C3H4N2 + CO2
The purity of CDI can be determined by the amount of CO2 that is formed upon hydrolysis (since the gas is formed essentially on a 1:1 molar ratio).
s, esters, and urea
s.
In the realm of peptide synthesis, this product may be treated with an amino acid or peptide ester (or amino acid hydrochloride in water) to release the imidazole group and couple the peptides. The side products, carbon dioxide and imidazole, are relatively innocuous. Racemization of the amino acid
s also tends to be minimal, due to mild reaction conditions.
CDI can also be used for esterification, although alcoholysis requires heat or the presence of a potent nucleophiles as sodium ethoxide,) and other strong bases like NaH. This reaction has generally good yield and wide scope (though forming the ester from tertiary alcohols when the acid reagent has a relatively acidic α-proton is troublesome, since C-C condensations
can occur, though this itself may be a desirable reaction). A similar reaction involving thiol
s and selenol
s can yield the corresponding esters. The alcohol reaction can be used to form glycosidic bonds, as well.
Similarly, an acid can be used in the place of an alcohol to form the anhydride. The equilibrium
is best shifted in the favor of the anhydride by utilizing an acid in a 2:1 ratio that forms an insoluble salt with the imidazole, such as trifluoro- or trichloroacetic acid
(and thus removes the free imidazole from the reaction). Symmetric anhydrides can thus be formed by replacing this trifluoro- or trichloroacetyl group with the acid that was used to form the original reagent.
Another related reaction is the reaction of formic acid
with CDI to form the formylized imidazole. This reagent is a good formylating agent
and can regenerate the unsubstituted imidazole (with formation of carbon monoxide) upon heating.
Yet another reaction involves the acylation of triphenylalkelynephosphoranes.
These can undergo the Wittig reaction
to form α,β unsaturated ketones or aldehydes.
The reagent can even undergo reaction with peroxide
to form the peroxycarboxylic acid, which can react further to form diacyl peroxides. The imidazole group is also reduced by LiAlH4 to form aldehydes from the carboxylic acid (rather than amines or alcohols). The reagent can also be reacted with Grignard reagents to form ketones.
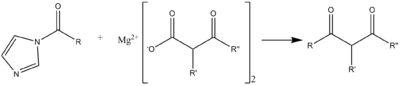
A C-C acylation reaction can occur with a malonic ester-type compound, in the following scheme useful for syntheses of macrolide antibiotics.
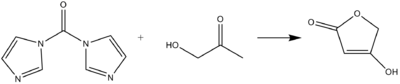
CDI can act as a carbonyl equivalent in the formation of tetronic acids or pulvinones from hydroxyketones and diketones in basic conditions.
An alcohol treated with at least 3 equivalents of an activated halide (such as allyl bromide or iodomethane) and CDI yields the corresponding bromide with good yield. Bromination and iodination work best, though this reaction does not preserve the stereochemistry
of the alcohol. In a similar context, CDI is often used in dehydration reactions.
As CDI is an equivalent of phosgene
, it can be used in similar reaction, however, with increased selectivity: it allows the synthesis of asymmetric bis alkyl carbonates
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the molecular formula (C3H3N2)2CO. It is a white crystalline solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
. It is often used for the coupling of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s for peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
synthesis and as a reagent in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Preparation
CDI can be prepared straightforwardly by the reaction of phosgenePhosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
with four equivalents of imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
under anhydrous conditions. Removal of the side product, imidazolium chloride, and solvent results in the crystalline product in ~90% yield.
- 4 C3H4N2ImidazoleImidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
+ C(O)Cl2 → (C3H3N2)2CO + 2 [C3H3N2H2]Cl
In this conversion, the imidazole serves both as the nucleophile and the base. An alternative precursor 1-(trimethylsilyl)imidazole requires more preparative effort with no corresponding advantages.
CDI hydrolyzes readily to give back imidazole:2CO + H2O → 2 C3H4N2 + CO2
The purity of CDI can be determined by the amount of CO2 that is formed upon hydrolysis (since the gas is formed essentially on a 1:1 molar ratio).
Use in synthesis
CDI is mainly employed to convert alcohols and amines into carbamateCarbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
s, esters, and urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
s.
Acid derivatives
One common extension of this scheme lies in the transacylation reaction of acids that is promoted by CDI. Although the reactivity of CDI is less than acid chlorides, it is more easily handled and its reactions have a wider scope in synthesis. An early application of this type of reaction was noted in the formation of imidazole peptide (and in general carboxylic acid) derivatives (with CO2 formation as a driving force).In the realm of peptide synthesis, this product may be treated with an amino acid or peptide ester (or amino acid hydrochloride in water) to release the imidazole group and couple the peptides. The side products, carbon dioxide and imidazole, are relatively innocuous. Racemization of the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s also tends to be minimal, due to mild reaction conditions.
CDI can also be used for esterification, although alcoholysis requires heat or the presence of a potent nucleophiles as sodium ethoxide,) and other strong bases like NaH. This reaction has generally good yield and wide scope (though forming the ester from tertiary alcohols when the acid reagent has a relatively acidic α-proton is troublesome, since C-C condensations
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
can occur, though this itself may be a desirable reaction). A similar reaction involving thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s and selenol
Selenol
Selenols are organic compounds that contain the functional group with the connectivity C-Se-H. Selenols are sometimes also called selenamercaptans, selenathiols, and selenothiols. Selenols are one of the principal classes of organoselenium compounds...
s can yield the corresponding esters. The alcohol reaction can be used to form glycosidic bonds, as well.
Similarly, an acid can be used in the place of an alcohol to form the anhydride. The equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
is best shifted in the favor of the anhydride by utilizing an acid in a 2:1 ratio that forms an insoluble salt with the imidazole, such as trifluoro- or trichloroacetic acid
Trichloroacetic acid
Trichloroacetic acid is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms....
(and thus removes the free imidazole from the reaction). Symmetric anhydrides can thus be formed by replacing this trifluoro- or trichloroacetyl group with the acid that was used to form the original reagent.
Another related reaction is the reaction of formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
with CDI to form the formylized imidazole. This reagent is a good formylating agent
Formylation reaction
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group .Aromatic formylation reactions via electrophilic aromatic substitution include:...
and can regenerate the unsubstituted imidazole (with formation of carbon monoxide) upon heating.
Yet another reaction involves the acylation of triphenylalkelynephosphoranes.
-
- (C6H5)3P=CHR + R'-CO-Im → (C6H5)3P+-CHR-COR' + Im-
(C6H5)3P+-CHR-COR' + (C6H5)3P=CHR → (C6H5)3P=CR-COR' + (C6H5)3P+-CH2R
- (C6H5)3P=CHR + R'-CO-Im → (C6H5)3P+-CHR-COR' + Im-
These can undergo the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
to form α,β unsaturated ketones or aldehydes.
The reagent can even undergo reaction with peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
to form the peroxycarboxylic acid, which can react further to form diacyl peroxides. The imidazole group is also reduced by LiAlH4 to form aldehydes from the carboxylic acid (rather than amines or alcohols). The reagent can also be reacted with Grignard reagents to form ketones.
A C-C acylation reaction can occur with a malonic ester-type compound, in the following scheme useful for syntheses of macrolide antibiotics.
Other reactions
The N-phenylimino derivative of CDI can be formed in a Wittig-like reaction.CDI can act as a carbonyl equivalent in the formation of tetronic acids or pulvinones from hydroxyketones and diketones in basic conditions.
An alcohol treated with at least 3 equivalents of an activated halide (such as allyl bromide or iodomethane) and CDI yields the corresponding bromide with good yield. Bromination and iodination work best, though this reaction does not preserve the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the alcohol. In a similar context, CDI is often used in dehydration reactions.
As CDI is an equivalent of phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
, it can be used in similar reaction, however, with increased selectivity: it allows the synthesis of asymmetric bis alkyl carbonates