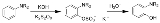
Boyland-Sims oxidation
Encyclopedia
The Boyland-Sims oxidation is the chemical reaction
of aniline
s with alkaline potassium persulfate, which after hydrolysis
forms ortho-hydroxyl anilines.
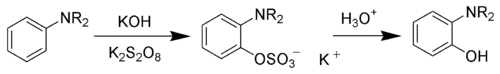 The ortho-isomer is formed predominantly. However, the para-sulfate is formed in small amounts with certain anilines.
The ortho-isomer is formed predominantly. However, the para-sulfate is formed in small amounts with certain anilines.
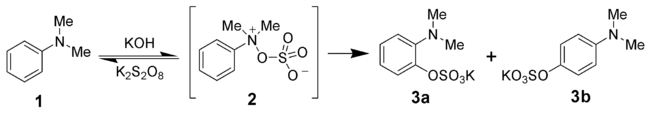
ic intermediate forms both the ortho- and para-amino aryl sulfates (3a and 3b).
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s with alkaline potassium persulfate, which after hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
forms ortho-hydroxyl anilines.

Reaction mechanism
Behrman has shown that the first intermediate in the Boyland-Sims oxidation is the formation of an arylhydroxylamine-O-sulfate (2). Rearrangement of this zwitterionZwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
ic intermediate forms both the ortho- and para-amino aryl sulfates (3a and 3b).


