
Boltzmann entropy
Encyclopedia
In thermodynamics
, specifically in statistical mechanics
, the Boltzmann entropy is an approximation to the normal Gibbs entropy.
The Boltzmann entropy is obtained if one assumes one can treat all the component particles of a thermodynamic system
as statistically independent. The probability distribution of the system as a whole then factorises into the product of N separate identical terms, one term for each particle; and the Gibbs entropy simplifies to the Boltzmann entropy
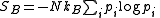
where the summation is taken over each possible state in the 6-dimensional phase space
of a single particle (rather than the 6N-dimensional phase space of the system as a whole).
This reflects the original statistical entropy function introduced by Ludwig Boltzmann
in 1872. For the special case of an ideal gas
it exactly corresponds to the proper thermodynamic entropy
.
However, for anything but the most dilute of real gases, it leads to increasingly wrong predictions of entropies and physical behaviours, by ignoring the interactions and correlations between different molecules. Instead one must follow Gibbs, and consider the ensemble of states of the system as a whole, rather than single particle states.
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, specifically in statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
, the Boltzmann entropy is an approximation to the normal Gibbs entropy.
The Boltzmann entropy is obtained if one assumes one can treat all the component particles of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
as statistically independent. The probability distribution of the system as a whole then factorises into the product of N separate identical terms, one term for each particle; and the Gibbs entropy simplifies to the Boltzmann entropy
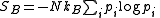
where the summation is taken over each possible state in the 6-dimensional phase space
Phase space
In mathematics and physics, a phase space, introduced by Willard Gibbs in 1901, is a space in which all possible states of a system are represented, with each possible state of the system corresponding to one unique point in the phase space...
of a single particle (rather than the 6N-dimensional phase space of the system as a whole).
This reflects the original statistical entropy function introduced by Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
in 1872. For the special case of an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
it exactly corresponds to the proper thermodynamic entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
.
However, for anything but the most dilute of real gases, it leads to increasingly wrong predictions of entropies and physical behaviours, by ignoring the interactions and correlations between different molecules. Instead one must follow Gibbs, and consider the ensemble of states of the system as a whole, rather than single particle states.
See also
- Entropy (thermodynamics)EntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- Boltzmann's entropy formulaBoltzmann's entropy formulaIn statistical thermodynamics, Boltzmann's equation is a probability equation relating the entropy S of an ideal gas to the quantity W, which is the number of microstates corresponding to a given macrostate:...
- Gibbs entropy

