
Biocatalysis
Encyclopedia
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task.
predates recorded history. The oldest records of brewing are about 6000 years old and refer to the Sumerians.
The employment of enzymes and whole cells have been important for many industries for centuries. The most obvious uses have been in the food and drink businesses where the production of wine, beer, cheese etc. is dependent on the effects of the microorganisms.
More than one hundred years ago, biocatalysis was employed to do chemical transformations on non-natural man-made organic compound
s, and the last 30 years have seen a substantial increase in the application of biocatalysis to produce fine chemicals, especially for the pharmaceutical industry.
Since biocatalysis deals with enzymes and microorganisms, it is historically classified separately from "homogeneous catalysis" and "heterogeneous catalysis". However, mechanistically speaking, biocatalysis is simply a special case of heterogeneous catalysis.
is selectivity
, which is necessary to obtain a high yield of a specific product. There are a large range of selective organic reaction
s available for most synthetic needs. However, there is still one area where organic chemists are struggling, and that is when chirality is involved, although considerable progress in chiral synthesis
has been achieved in recent years.
Enzymes display three major types of selectivities:
These reasons, and especially the latter, are the major reasons why synthetic chemists have become interested in biocatalysis. This interest in turn is mainly due to the need to synthesise enantiopure compounds as chiral building blocks for drugs
and agrochemicals.
Another important advantage of biocatalysts are that they are environmentally acceptable, being completely degraded in the environment. Furthermore the enzymes act under mild conditions, which minimizes problems of undesired side-reactions such as decomposition, isomerization, racemization
and rearrangement
, which often plague traditional methodology.
In kinetic resolution
of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the enantiomers into product at a greater reaction rate
than the other enantiomer.
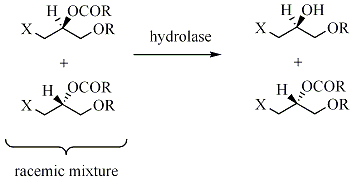 The racemic mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology. The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower enantiomeric excess
The racemic mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology. The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower enantiomeric excess
. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called dynamic resolution.
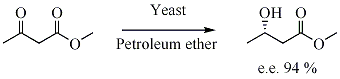
In biocatalysed asymmetric synthesis, a non-chiral unit becomes chiral in such a way that the different possible stereoisomers are formed in different quantities. The chirality is introduced into the substrate by influence of enzyme, which is chiral. Yeast
is a biocatalyst for the enantioselective reduction of ketone
s.
 The biocatalytic Baeyer-Villiger oxidation
The biocatalytic Baeyer-Villiger oxidation
is another example of a biocatalytic reaction. In one study a specially designed mutant of Candida Antarctica
was found to be an effective catalyst for the Michael addition of acrolein
with acetylacetone
at 20 °C in absence of additional solvent.
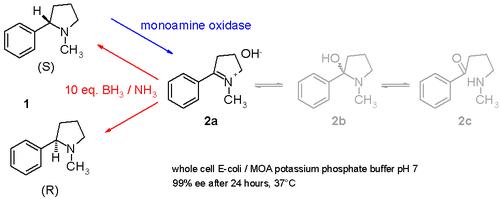
Another study demonstrates how racemic nicotine
(mixture of S and R-enantiomers 1 in scheme 3) can be deracemized in a one-pot
procedure involving a monoamine oxidase isolated from Aspergillus niger
which is able to oxidize only the amine
S-enantiomer to the imine
2 and involving an ammonia
–borane
reducing
couple which can reduce the imine 2 back to the amine 1. In this way the S-enantiomer will continuously be consumed by the enzyme while the R-enantiomer accumulates. It is even possible to stereoinvert pure S to pure R.
History
Biocatalysis underpins some of the oldest chemical transformations known to humans, for brewingBrewing
Brewing is the production of beer through steeping a starch source in water and then fermenting with yeast. Brewing has taken place since around the 6th millennium BCE, and archeological evidence suggests that this technique was used in ancient Egypt...
predates recorded history. The oldest records of brewing are about 6000 years old and refer to the Sumerians.
The employment of enzymes and whole cells have been important for many industries for centuries. The most obvious uses have been in the food and drink businesses where the production of wine, beer, cheese etc. is dependent on the effects of the microorganisms.
More than one hundred years ago, biocatalysis was employed to do chemical transformations on non-natural man-made organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s, and the last 30 years have seen a substantial increase in the application of biocatalysis to produce fine chemicals, especially for the pharmaceutical industry.
Since biocatalysis deals with enzymes and microorganisms, it is historically classified separately from "homogeneous catalysis" and "heterogeneous catalysis". However, mechanistically speaking, biocatalysis is simply a special case of heterogeneous catalysis.
Advantages
The key word for organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
is selectivity
Chemoselectivity
Chemical reactions are defined usually in small contexts , such generalizations are a matter of utility. The preferential outcome of one instance of a generalized reaction over a set of other plausible reactions, is defined as chemoselectivity...
, which is necessary to obtain a high yield of a specific product. There are a large range of selective organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s available for most synthetic needs. However, there is still one area where organic chemists are struggling, and that is when chirality is involved, although considerable progress in chiral synthesis
Chiral synthesis
Enantioselective synthesis, also called chiral synthesis, asymmetric synthesis or stereoselective synthesis, is organic synthesis that introduces one or more new and desired elements of chirality...
has been achieved in recent years.
Enzymes display three major types of selectivities:
- ChemoselectivityChemoselectivityChemical reactions are defined usually in small contexts , such generalizations are a matter of utility. The preferential outcome of one instance of a generalized reaction over a set of other plausible reactions, is defined as chemoselectivity...
: Since the purpose of an enzyme is to act on a single type of functional groupFunctional groupIn organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, other sensitive functionalities, which would normally react to a certain extent under chemical catalysis, survive. As a result, biocatalytic reactions tend to be "cleaner" and laborious purification of product(s) from impurities emerging through side-reactions can largely be omitted. - RegioselectivityRegioselectivityIn chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
and diastereoselectivity: Due to their complex three-dimensional structure, enzymes may distinguish between functional groups which are chemically situated in different regions of the substrate molecule. - Enantioselectivity: Since almost all enzymes are made from L-amino acidAmino acidAmino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, enzymes are chiral catalysts. As a consequence, any type of chirality present in the substrate molecule is "recognized" upon the formation of the enzyme-substrate complex. Thus a prochiral substrate may be transformed into an optically active product and both enantiomers of a racemic substrate may react at different rates.
These reasons, and especially the latter, are the major reasons why synthetic chemists have become interested in biocatalysis. This interest in turn is mainly due to the need to synthesise enantiopure compounds as chiral building blocks for drugs
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
and agrochemicals.
Another important advantage of biocatalysts are that they are environmentally acceptable, being completely degraded in the environment. Furthermore the enzymes act under mild conditions, which minimizes problems of undesired side-reactions such as decomposition, isomerization, racemization
Racemization
In chemistry, racemization refers to the converting of an enantiomerically pure mixture into a mixture where more than one of the enantiomers are present...
and rearrangement
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
, which often plague traditional methodology.
Asymmetric biocatalysis
The use of biocatalysis to obtain enantiopure compounds can be divided into two different methods:- Kinetic resolution of a racemic mixture
- Biocatalysed asymmetric synthesis
In kinetic resolution
Kinetic resolution
In kinetic resolution, two enantiomers show different reaction rates in a chemical reaction, thereby creating an excess of the less reactive enantiomer. This excess goes through a maximum and disappears on full completion of the reaction. Kinetic resolution is a very old concept in organic...
of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the enantiomers into product at a greater reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
than the other enantiomer.

Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called dynamic resolution.
In biocatalysed asymmetric synthesis, a non-chiral unit becomes chiral in such a way that the different possible stereoisomers are formed in different quantities. The chirality is introduced into the substrate by influence of enzyme, which is chiral. Yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
is a biocatalyst for the enantioselective reduction of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s.

Baeyer-Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction in which a ketone is oxidized to an ester by treatment with peroxy acids or hydrogen peroxide. Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry...
is another example of a biocatalytic reaction. In one study a specially designed mutant of Candida Antarctica
Candida (genus)
Candida is a genus of yeasts. Many species are harmless commensals or endosymbionts of animal hosts including humans, but other species, or harmless species in the wrong location, can cause disease. Candida albicans can cause infections in humans and other animals, especially in immunocompromised...
was found to be an effective catalyst for the Michael addition of acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
with acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
at 20 °C in absence of additional solvent.
Another study demonstrates how racemic nicotine
Nicotine
Nicotine is an alkaloid found in the nightshade family of plants that constitutes approximately 0.6–3.0% of the dry weight of tobacco, with biosynthesis taking place in the roots and accumulation occurring in the leaves...
(mixture of S and R-enantiomers 1 in scheme 3) can be deracemized in a one-pot
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
procedure involving a monoamine oxidase isolated from Aspergillus niger
Aspergillus niger
Aspergillus niger is a fungus and one of the most common species of the genus Aspergillus. It causes a disease called black mold on certain fruits and vegetables such as grapes, onions, and peanuts, and is a common contaminant of food...
which is able to oxidize only the amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
S-enantiomer to the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
2 and involving an ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
–borane
Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
reducing
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
couple which can reduce the imine 2 back to the amine 1. In this way the S-enantiomer will continuously be consumed by the enzyme while the R-enantiomer accumulates. It is even possible to stereoinvert pure S to pure R.

External links
- The University of Exeter - Biocatalysis Centre
- Applied Biocatalysis centre - Graz
- Center for Biocatalysis and Bioprocessing - The University of Iowa
- TU Delft - Biocatalysis & Organic Chemistry (BOC)
- KTH Stockholm - Biocatalysis Research Group
- MIT Short Course - Principles and Applications of Biocatalysis
- Web Resource for biocatalysis and enzymes

