
Ammonium metavanadate
Encyclopedia
Ammonium metavanadate, NH4VO3, is a yellow crystalline solid which is water soluble inorganic acid that acts as insulin
mimic. It functions as a catalyst to certain reactions and is known to have toxic effects in certain species. It has been found in slags and fly ash from coal burning which has been known to cause respiratory problems. Ammonium metavanadate belongs to the family of vanadates that contains many different oxidative states that are dependent on pH. Ammonium metavanadate is the most common laboratory vanadate reagent, along with potassium metavanadate, KVO3.
Ammonium metavanadate administration in rats has been known to have negative effects on fertility, reproductive performance, and progeny. Ammonium metavanadate and other vanadium compounds are “known to cross the placental barrier and accumulate in the fetuses especially in the fetal skeleton.” In a study by Morgan and El-Tawil, the effects of ammonium metavanadate was examined in three groups; control without ammonium metavanadate, treated female rats with untreated males, and untreated female rats with treated males. It was noted that ammonium metavanadate caused visceral and skeletal anomalies in both treated groups. In fetuses, visceral anomalies consisted of dilated brain ventricles, dilated nares, olfactory bulb, renal hypoplasia, and other abnormalities. In fetuses, skeletal anomalies consisted of wide separation of parietal bones, extra ribs, absence of carpal and metacarpal, tarsal and metatarsal bones, and other abnormalities. Reproductive organs of the treated sex were also shown to be affected and to weigh less than those of untreated rats. No overall body weight was seen in treated rats.
A few studies have been done to counteract the toxic effects of ammonium metavanadate. The study by Soussi et al., examined the cytotoxicity caused by oxidative stress from indigestion of ammonium metavanadate in Wistar rats and the anti-oxidative effects of Camillia sinensis tea leaves after the administration of ammonium metavanadate. Camillia sinensis was selected for its “polyphenols which are known to possess antioxidative properties due to their radical scavenging and metal-chelating functions.” From the analysis of the impact and effects of lipid peroxidation, superoxidismutase, catalase activities, vitamin E, and vitamin A, both oxidative stress properties of ammonium metavanadate and anti-oxidative properties of the Camillia sinensis were supported.
Ammonium metavanadate has been identified in fly ash from coal combustion which has been known to cause respiratory problems. A study by Vaddi and Wei, demonstratred the effects of ammonium metavanadate on bovine pulmonary alveolar macrophages. These macrophages were selected to demonstrate to effect of ammonium metavanadate on the respiratory system’s defense mechanisms. Viability, phagocytosis, and chemiluminesce (with the presence of luminol) of macrophages were examined with the presence of various concentrations, temperatures, and exposure time. Cytotoxicity was found in high concentrations (0.5-1.0 µl/ml) after 8 hours based on phagocytic activity and viability test. Low concentrations of ammonium metavanadate (0.01-0.1 µl/ml) were not cytotoxic “even after 24 hour exposure”. Cytotoxicity within these macrophages can lead to infection and disease of the respiratory system.
(DTA) curves. Catalytic activity was measured “using hydrogen peroxide decomposition at 30, 40, and 50°C” for pure and mixed compounds. Pure ammonium metavanadate and basic copper carbonate exhibited very small catalytic activity at 300 and 500°C. Catalytic activity of the mixed molar ratios increased with the increase of ammonium metavanadate. No catalytic activity occurred at 750 and 1000° C for either pure or mixed. Shaheen and Maksod, believed that the incr ease in catalytic activity was due to “the increase in the concentration of active sites by creation of new ion pairs… as a result of mutual charge interaction” and no catalytic activity of 750 and 1000°C was due to “restrict ion of catalytically active constituents by the formation of copper vanadate compounds”. Monitoring of activation energy provided evidence that “the addition of increasing amounts of vanadium oxide resulted in a measurable decrease in” activation energy. The ESR spectra data from this study suggests that the catalytic activity is based on “the concept of bivalent catalytic centers.”
activity in humans hepatocytes through the analysis of urine after the administration of isoniazide and ammonium metavanadate. N-Acetyltransferase is important because it acts as an important enzyme of drug metabolism. NH4VO3, forms a ligand complex with isoniazide and ligand formation happens at nitrogen and oxygen with V(V) as the metal. The interaction is dependent on the pH and the amount of AMV present. Higher acidity causes absorption intensity and the higher the concentration of complex formation. Lower pH decreases the content of VO2+ in solution and increases content of dioxohydroxovanadium(V) anion (VO2OH) and hexadecaoxohexavanadate(V) anion (V6O162-). This causes the complex formation to be more complete.
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
mimic. It functions as a catalyst to certain reactions and is known to have toxic effects in certain species. It has been found in slags and fly ash from coal burning which has been known to cause respiratory problems. Ammonium metavanadate belongs to the family of vanadates that contains many different oxidative states that are dependent on pH. Ammonium metavanadate is the most common laboratory vanadate reagent, along with potassium metavanadate, KVO3.
Crystal structure
Crystalline ammonium metavanadate contains infinitely long chains of corner-sharing VO4 tetrahedra.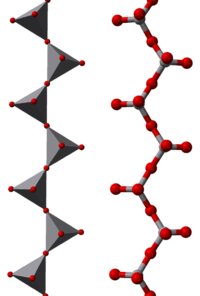 |
||
Ball-and-stick model In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them... |
Pharmacology
Many pharmacology studies have been performed to understand the effects of ammonium metavanadate. Studies have been performed on Piearactus mesopotamicus, Wistar rats, bovine, murine, and Samonella typhimurium. In the study of Holmberg, different doses of ammonium metavanadate were tested and examined for growth and metabolism effects on pacu. The study did not improve the metabolism of as hypothesized but cytotoxicity and reduced growth was noted at high doses.Ammonium metavanadate administration in rats has been known to have negative effects on fertility, reproductive performance, and progeny. Ammonium metavanadate and other vanadium compounds are “known to cross the placental barrier and accumulate in the fetuses especially in the fetal skeleton.” In a study by Morgan and El-Tawil, the effects of ammonium metavanadate was examined in three groups; control without ammonium metavanadate, treated female rats with untreated males, and untreated female rats with treated males. It was noted that ammonium metavanadate caused visceral and skeletal anomalies in both treated groups. In fetuses, visceral anomalies consisted of dilated brain ventricles, dilated nares, olfactory bulb, renal hypoplasia, and other abnormalities. In fetuses, skeletal anomalies consisted of wide separation of parietal bones, extra ribs, absence of carpal and metacarpal, tarsal and metatarsal bones, and other abnormalities. Reproductive organs of the treated sex were also shown to be affected and to weigh less than those of untreated rats. No overall body weight was seen in treated rats.
A few studies have been done to counteract the toxic effects of ammonium metavanadate. The study by Soussi et al., examined the cytotoxicity caused by oxidative stress from indigestion of ammonium metavanadate in Wistar rats and the anti-oxidative effects of Camillia sinensis tea leaves after the administration of ammonium metavanadate. Camillia sinensis was selected for its “polyphenols which are known to possess antioxidative properties due to their radical scavenging and metal-chelating functions.” From the analysis of the impact and effects of lipid peroxidation, superoxidismutase, catalase activities, vitamin E, and vitamin A, both oxidative stress properties of ammonium metavanadate and anti-oxidative properties of the Camillia sinensis were supported.
Ammonium metavanadate has been identified in fly ash from coal combustion which has been known to cause respiratory problems. A study by Vaddi and Wei, demonstratred the effects of ammonium metavanadate on bovine pulmonary alveolar macrophages. These macrophages were selected to demonstrate to effect of ammonium metavanadate on the respiratory system’s defense mechanisms. Viability, phagocytosis, and chemiluminesce (with the presence of luminol) of macrophages were examined with the presence of various concentrations, temperatures, and exposure time. Cytotoxicity was found in high concentrations (0.5-1.0 µl/ml) after 8 hours based on phagocytic activity and viability test. Low concentrations of ammonium metavanadate (0.01-0.1 µl/ml) were not cytotoxic “even after 24 hour exposure”. Cytotoxicity within these macrophages can lead to infection and disease of the respiratory system.
Catalytic activity
Ammonium metavanadate acts as a catalyst for various reactions. It has been identified as a catalyst for “α-hydroxyphosphonate derivatives by the reaction of various aryl or heteroaryl aldehydes with triethylphosphonate derivatives.” The end product of these reactions, hydroxyphosphonates, are important because they “act as an inhibitor of a diverse group of enzymes including Renin, FPTase, HIV protease, and EPSP synthase” and “show antibacterial activity with the quinoline nucleus.” Ammonium metavanadate is environmentally friendly compared to past organic solvents used as catalysts in these and similar reactions. In the study of Sonar et al., ammonium metavanadate assisted the reaction of triethyl phosphate with 2-chlororquinoline-3-carbaldehyde to produce the α-hydroxyphosphosphonates. It was determined to produce a high yield of 94% with a much shorter reaction time of 5 minutes compared to both its organic solvents and organic solvent free counterparts.Metallurgy
Ammonium metavanadate has been mixed with basic copper carbonate to make a new compound with better catalytic activity than the two separate compounds. In the study by Shaheen and Maksod, catalytic activity of different molar ratios of ammonium metavanadate in comparison with the two original compounds was examined at different calcinations temperatures. Crystalline structures and composition of compounds at certain temperatures were identified by X-ray diffraction and Differential thermal analysisDifferential thermal analysis
Differential thermal analysis is a thermoanalytic technique, similar to differential scanning calorimetry. In DTA, the material under study and an inert reference are made to undergo identical thermal cycles, while recording any temperature difference between sample and reference...
(DTA) curves. Catalytic activity was measured “using hydrogen peroxide decomposition at 30, 40, and 50°C” for pure and mixed compounds. Pure ammonium metavanadate and basic copper carbonate exhibited very small catalytic activity at 300 and 500°C. Catalytic activity of the mixed molar ratios increased with the increase of ammonium metavanadate. No catalytic activity occurred at 750 and 1000° C for either pure or mixed. Shaheen and Maksod, believed that the incr ease in catalytic activity was due to “the increase in the concentration of active sites by creation of new ion pairs… as a result of mutual charge interaction” and no catalytic activity of 750 and 1000°C was due to “restrict ion of catalytically active constituents by the formation of copper vanadate compounds”. Monitoring of activation energy provided evidence that “the addition of increasing amounts of vanadium oxide resulted in a measurable decrease in” activation energy. The ESR spectra data from this study suggests that the catalytic activity is based on “the concept of bivalent catalytic centers.”
Sensor
Ammonium metavanadate has the ability to act as a sensor for N-AcetyltransferaseN-Acetyltransferase
N-acetyltransferase is an enzyme that catalyzes the transfer of acetyl groups from acetyl-CoA to arylamines. They have wide specificity for aromatic amines, particularly serotonin, and can also catalyze acetyl transfer between arylamines without CoA. EC 2.3.1.5....
activity in humans hepatocytes through the analysis of urine after the administration of isoniazide and ammonium metavanadate. N-Acetyltransferase is important because it acts as an important enzyme of drug metabolism. NH4VO3, forms a ligand complex with isoniazide and ligand formation happens at nitrogen and oxygen with V(V) as the metal. The interaction is dependent on the pH and the amount of AMV present. Higher acidity causes absorption intensity and the higher the concentration of complex formation. Lower pH decreases the content of VO2+ in solution and increases content of dioxohydroxovanadium(V) anion (VO2OH) and hexadecaoxohexavanadate(V) anion (V6O162-). This causes the complex formation to be more complete.

