
ATP hydrolysis
Encyclopedia
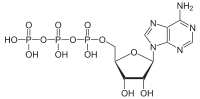
ATP hydrolysis is the reaction by which chemical energy that has been stored and transported in the high-energy phosphoanhydridic bonds
in ATP
(Adenosine triphosphate) is released, for example in the muscles, to produce work. The product is ADP
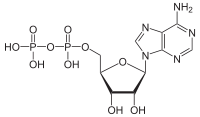
(Adenosine diphosphate) and an inorganic phosphate, orthophosphate (Pi). ADP can be further hydrolyzed to give energy, AMP
(Adenosine monophosphate), and another orthophosphate (Pi). ATP hydrolysis is the final link between the energy derived from food or sunlight and useful work such as muscle contraction, the establishment of ion gradients across membranes, and biosynthetic processes necessary to maintain life.
The description and typical textbook labeling of ATP phosphanhydridic bonds as "high energy . . bonds" can be very misleading to students. These bonds are in fact relatively weak. They do involve high energy electrons but the bonds themselves are quite easy to break. As noted below, energy is released by the hydrolysis of ATP when these weak bonds are broken - requiring a small input of energy, followed by the formation of new bonds and the release of a larger amount of energy as the total energy of the system is lowered and becomes more stable.
Hydrolysis
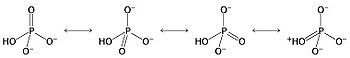
of the phosphate
groups in ATP is especially exergonic
, because the resulting orthophosphate group is greatly stabilized by multiple resonance structures, making the products (ADP and Pi) much lower in energy than the reactant (ATP). The negative charge density associated with the three adjacent phosphate units of ATP also destabilizes the molecule, making it higher in energy. Hydrolysis relieves some of these electrostatic repulsions as well, liberating useful energy in the process.
Hydrolysis of the terminal phosphoanhydridic bond is a highly exergonic process, releasing -30.5 kJ mol−1 energy. This reaction can then be coupled with thermodynamically unfavorable reactions to give an overall negative (spontaneous) ΔG for the reaction sequence. The actual value of ΔG for ATP hydrolysis varies, primarily depending on Mg2+ concentration, and under normal physiologic conditions is actually closer to -50 kJ mol−1.
In humans, approximately 60 percent of the energy released from the hydrolysis of one mole
of ATP produces metabolic heat rather than fuel the actual reactions taking place.
Due to the acid-base properties of ATP, ADP, and inorganic phosphate, the hydrolysis of ATP has the effect of lowering the pH of the reaction medium. Under certain conditions, high levels of ATP hydrolysis can contribute to lactic acidosis
.
High energy phosphate
High-energy phosphate can mean one of two things:* The phosphate-phosphate bonds formed when compounds such as adenosine diphosphate and adenosine triphosphate are created....
in ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(Adenosine triphosphate) is released, for example in the muscles, to produce work. The product is ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(Adenosine diphosphate) and an inorganic phosphate, orthophosphate (Pi). ADP can be further hydrolyzed to give energy, AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
(Adenosine monophosphate), and another orthophosphate (Pi). ATP hydrolysis is the final link between the energy derived from food or sunlight and useful work such as muscle contraction, the establishment of ion gradients across membranes, and biosynthetic processes necessary to maintain life.
The description and typical textbook labeling of ATP phosphanhydridic bonds as "high energy . . bonds" can be very misleading to students. These bonds are in fact relatively weak. They do involve high energy electrons but the bonds themselves are quite easy to break. As noted below, energy is released by the hydrolysis of ATP when these weak bonds are broken - requiring a small input of energy, followed by the formation of new bonds and the release of a larger amount of energy as the total energy of the system is lowered and becomes more stable.
Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
groups in ATP is especially exergonic
Exergonic
Exergonic means "releasing energy in the form of work". By thermodynamic standards, work, a form of energy, is defined as moving from the system to the surroundings...
, because the resulting orthophosphate group is greatly stabilized by multiple resonance structures, making the products (ADP and Pi) much lower in energy than the reactant (ATP). The negative charge density associated with the three adjacent phosphate units of ATP also destabilizes the molecule, making it higher in energy. Hydrolysis relieves some of these electrostatic repulsions as well, liberating useful energy in the process.
Hydrolysis of the terminal phosphoanhydridic bond is a highly exergonic process, releasing -30.5 kJ mol−1 energy. This reaction can then be coupled with thermodynamically unfavorable reactions to give an overall negative (spontaneous) ΔG for the reaction sequence. The actual value of ΔG for ATP hydrolysis varies, primarily depending on Mg2+ concentration, and under normal physiologic conditions is actually closer to -50 kJ mol−1.
In humans, approximately 60 percent of the energy released from the hydrolysis of one mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of ATP produces metabolic heat rather than fuel the actual reactions taking place.
Due to the acid-base properties of ATP, ADP, and inorganic phosphate, the hydrolysis of ATP has the effect of lowering the pH of the reaction medium. Under certain conditions, high levels of ATP hydrolysis can contribute to lactic acidosis
Lactic acidosis
Lactic acidosis is a physiological condition characterized by low pH in body tissues and blood accompanied by the buildup of lactate especially D-lactate, and is considered a distinct form of metabolic acidosis. The condition typically occurs when cells receive too little oxygen , for example...
.




