
2-oxopent-4-enoate hydratase
Encyclopedia
In enzymology, a 2-oxopent-4-enoate hydratase is an enzyme
that catalyzes
the chemical reaction
Hence, this enzyme has one substrate
, 4-hydroxy-2-oxopentanoate, and two products
, 2-oxopent-4-enoate and H2O
.
This enzyme belongs to the family of lyase
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is 4-hydroxy-2-oxopentanoate hydro-lyase (2-oxopent-4-enoate-forming). Other names in common use include 2-keto-4-pentenoate hydratase, OEH, 2-keto-4-pentenoate (vinylpyruvate)hydratase, and 4-hydroxy-2-oxopentanoate hydro-lyase. This enzyme participates in 9 metabolic pathways
: phenylalanine metabolism, benzoate degradation via hydroxylation, biphenyl degradation, toluene and xylene degradation, 1,4-dichlorobenzene degradation, fluorene degradation, carbazole degradation, ethylbenzene degradation, and styrene degradation.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- 4-hydroxy-2-oxopentanoate
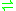 2-oxopent-4-enoate + H2O
2-oxopent-4-enoate + H2O
Hence, this enzyme has one substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
, 4-hydroxy-2-oxopentanoate, and two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
, 2-oxopent-4-enoate and H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
.
This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is 4-hydroxy-2-oxopentanoate hydro-lyase (2-oxopent-4-enoate-forming). Other names in common use include 2-keto-4-pentenoate hydratase, OEH, 2-keto-4-pentenoate (vinylpyruvate)hydratase, and 4-hydroxy-2-oxopentanoate hydro-lyase. This enzyme participates in 9 metabolic pathways
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
: phenylalanine metabolism, benzoate degradation via hydroxylation, biphenyl degradation, toluene and xylene degradation, 1,4-dichlorobenzene degradation, fluorene degradation, carbazole degradation, ethylbenzene degradation, and styrene degradation.

