2,3-Bisphosphoglycerate
Encyclopedia
2,3-Bisphosphoglyceric acid (2,3-Bisphosphoglycerate or 2,3-BPG, also known as 2,3-diphosphoglycerate or 2,3-DPG) is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid (1,3-BPG). 2,3-BPG is present in human red blood cells (RBC; erythrocyte) at approximately 5 mmol/L. It binds with greater affinity to deoxygenated hemoglobin
(e.g. when the red cell is near respiring tissue) than it does to oxygenated hemoglobin (e.g., in the lungs) due to spatial changes: 2,3-BPG (whose size is estimated at about 9 angstroms) fits in the deoxygenated hemoglobin configuration (11 angstroms), but not as well in the oxygenated (5 angstroms). It interacts with deoxygenated hemoglobin beta subunits by decreasing their affinity for oxygen, so it allosterically promotes the release of the remaining oxygen molecules bound to the hemoglobin, thus enhancing the ability of RBCs to release oxygen near tissues that need it most. 2,3-BPG is thus an allosteric effector.
Its function was discovered in 1967 by Reinhold Benesch and Ruth Benesch.
. It can then be broken down by 2,3-BPG phosphatase
to form 3-phosphoglycerate. Its synthesis and breakdown are, therefore, a way around a step of glycolysis
.
Erythrocytes synthesize and degrade the 2.3-BPG by a diversion of the glycolytic pathway.
The first phase of glucose catabolism includes glucose phosphorylation, isomerization and another phosphorylation to bear fructose-1,6-bisphosphate (F-1,6-BP). Cleavage of fructose 1, 6-bisphosphate yields two molecules: glyceraldehyde 3-phosphate (G3P and DHAP respectively)and dihydroxyacetone 3 phosphate. These two molecules are isomers and are readily converterd into one another by triose phosphate isomerase. The equilibrium of this conversion lies heavily on the side of DHAP for two reasons. Firstly so the glycolytic pathway does not get oversaturated and secondly so that the biochemistry of glycerol can be tied into glycolysis. DHAP can be converted into glycerol when the supply of DHAP is plentiful and glycerol can then be used as a substrate for phase 2 glycolysis when glycolytic intermediates are scarce.
The second phase of glucose catabolism converts G3P to 3-phophoglycerate (3-PG). During the first reaction step, G3P is phosphorylated with a high-energy phosphate and oxidized to 1,3-bisphosphoglycerate (1,3-BPG), through the action of glyceralgehyde-3-phosphate dehydrogenase (G3PD). 1,3-BPG may be dephosphorylated by phosphoglycerate kinase (PGK), generating ATP, or it may be shunted into the Luebering-Rapapport pathway, where bisphosphoglycerate mutase catalyzes the transfer of a phosphoryl group from C1 to C2 of 1,3-BPG, giving 2,3-BPG. 2,3-BPG, the most concentrated organophosphate in the erythrocyte, forms 3-PG by the action of diphosphoglycerate phosphatase. The concentration on 2,3-BPG varies inversely with the pH, which is inhibitory to catalytic action of bisphosphoglyceromutase.
The third phase of anaerobic glucose catabolism involves conversion of 3-PG to pyruvate with the generation of ATP.
There is a delicate balance between the need to generate ATP
to support energy requirements for cell metabolism and the need to maintain appropriate oxygenation/deoxygenation status of hemoglobin. This balance is maintained by dephosphorilation of 1,3-BPG to 2,3-BPG, which enhances the deoxygenation of hemoglobin. Low pH inhibits the activity of biphosphoglyceromutase and activates bisphosphoglyerate phosphatase, which favors generation of ATP.
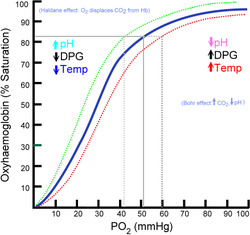 When 2,3-BPG binds to deoxyhemoglobin, it acts to stabilize the low oxygen affinity state (T state) of the oxygen carrier. It fits neatly into the cavity of the deoxy- conformation, exploiting the molecular symmetry
When 2,3-BPG binds to deoxyhemoglobin, it acts to stabilize the low oxygen affinity state (T state) of the oxygen carrier. It fits neatly into the cavity of the deoxy- conformation, exploiting the molecular symmetry
and positive polarity by forming salt bridges with lysine
and histidine
residues in the four subunits of hemoglobin
. The R state, with oxygen bound to a heme group, has a different conformation and does not allow this interaction. By itself, hemoglobin has sigmoid-like kinetics, which makes easier another subunits’ binding (the first molecule of oxygen helps the following to link).
By selectively binding to deoxyhemoglobin, 2,3-BPG stabilizes the T state conformation, making it harder for oxygen to bind hemoglobin and more likely to be released to adjacent tissues. 2,3-BPG is part of a feedback loop that can help prevent tissue hypoxia
in conditions where it is most likely to occur. Conditions of low tissue oxygen concentration such as high altitude (2,3-BPG levels are higher in those acclimated to high altitudes), airway obstruction
, or congestive heart failure
will tend to cause RBCs to generate more 2,3-BPG in their effort to generate energy by allowing more oxygen to be released in tissues deprived of oxygen. Ultimately, this mechanism increases oxygen release from RBCs under circumstances where it is needed most. This release is potentiated by the Bohr effect
in tissues with high energetic demands. Bohr effect is another useful way to solve the affinity problem of the hemoglobin, and it’s related to the pH and the CO2.
It’s important to highlight that the behaviour of myoglobin
doesn’t work in the same way, as 2,3-BPG has no effect on it.
(HbF) exhibits a low affinity for 2,3-BPG, resulting in a higher binding affinity for oxygen. This increased oxygen-binding affinity relative to that of adult hemoglobin (HbA) is due to HbF's having two α/γ dimers as opposed to the two α/β dimers of HbA. The positive histidine
residues of HbA β-subunits that are essential for forming the 2,3-BPG binding pocket are replaced by serine
residues in HbF γ-subunits. Like that, histidine nº143 gets lost, so 2,3-BPG has difficulties in linking to the fetal hemoglobin, and it looks like the pure hemoglobin.
That’s the way O2 flows from the mother to the fetus.
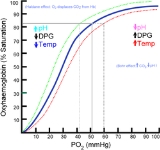
As we can see in the following image, fetal hemoglobin has more affinity to oxygen than adult hemoglobin. Moreover, myoglobin has the highest affinity to oxygen.

Differences between myoglobin (Mb), fetal hemoglobin (Hb F), adult hemoglobin (Hb A)
A 2004 study checked the effects of thyroid hormone on 2,3-BPG levels. The result was that the hyperthyroidism modulates in vivo 2,3-BPG content in erythrocytes by changes in the expression of phosphoglycerate mutase
(PGM) and 2,3-BPG synthase.
This result shows that the increase in the 2,3-BPG content of erythrocytes observed in hyperthyroidism doesn’t depend on any variation in the rate of circulating hemoglobin, but seems to be a direct consequence of the stimulating effect of thyroid hormones on erythrocyte glycolytic activity.
This illness is characterized by a lack of iron, and as 2,3-BPG needs this chemical element to be synthesized, BPG concentration decreases and hemoglobin binds tightly to oxygen. As a result, oxygen release to tissue is reduced.
Recently, scientists have found similarities between low amounts of 2,3-BPG with the occurrence of high altitude pulmonary edema at high altitudes.
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
(e.g. when the red cell is near respiring tissue) than it does to oxygenated hemoglobin (e.g., in the lungs) due to spatial changes: 2,3-BPG (whose size is estimated at about 9 angstroms) fits in the deoxygenated hemoglobin configuration (11 angstroms), but not as well in the oxygenated (5 angstroms). It interacts with deoxygenated hemoglobin beta subunits by decreasing their affinity for oxygen, so it allosterically promotes the release of the remaining oxygen molecules bound to the hemoglobin, thus enhancing the ability of RBCs to release oxygen near tissues that need it most. 2,3-BPG is thus an allosteric effector.
Its function was discovered in 1967 by Reinhold Benesch and Ruth Benesch.
Metabolism
2,3-BPG is formed from 1,3-BPG by the enzyme 2,3-BPG mutaseBisphosphoglycerate mutase
Bisphosphoglycerate mutase is an enzyme unique to erythrocytes and placental cells. It is responsible for the catalytic synthesis of 2,3-Bisphosphoglycerate from 1,3-bisphosphoglycerate...
. It can then be broken down by 2,3-BPG phosphatase
Bisphosphoglycerate phosphatase
In enzymology, a bisphosphoglycerate phosphatase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are 2,3-bisphospho-D-glycerate and H2O, whereas its two products are 3-phospho-D-glycerate and phosphate....
to form 3-phosphoglycerate. Its synthesis and breakdown are, therefore, a way around a step of glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
.
Erythrocytes synthesize and degrade the 2.3-BPG by a diversion of the glycolytic pathway.
The first phase of glucose catabolism includes glucose phosphorylation, isomerization and another phosphorylation to bear fructose-1,6-bisphosphate (F-1,6-BP). Cleavage of fructose 1, 6-bisphosphate yields two molecules: glyceraldehyde 3-phosphate (G3P and DHAP respectively)and dihydroxyacetone 3 phosphate. These two molecules are isomers and are readily converterd into one another by triose phosphate isomerase. The equilibrium of this conversion lies heavily on the side of DHAP for two reasons. Firstly so the glycolytic pathway does not get oversaturated and secondly so that the biochemistry of glycerol can be tied into glycolysis. DHAP can be converted into glycerol when the supply of DHAP is plentiful and glycerol can then be used as a substrate for phase 2 glycolysis when glycolytic intermediates are scarce.
The second phase of glucose catabolism converts G3P to 3-phophoglycerate (3-PG). During the first reaction step, G3P is phosphorylated with a high-energy phosphate and oxidized to 1,3-bisphosphoglycerate (1,3-BPG), through the action of glyceralgehyde-3-phosphate dehydrogenase (G3PD). 1,3-BPG may be dephosphorylated by phosphoglycerate kinase (PGK), generating ATP, or it may be shunted into the Luebering-Rapapport pathway, where bisphosphoglycerate mutase catalyzes the transfer of a phosphoryl group from C1 to C2 of 1,3-BPG, giving 2,3-BPG. 2,3-BPG, the most concentrated organophosphate in the erythrocyte, forms 3-PG by the action of diphosphoglycerate phosphatase. The concentration on 2,3-BPG varies inversely with the pH, which is inhibitory to catalytic action of bisphosphoglyceromutase.
The third phase of anaerobic glucose catabolism involves conversion of 3-PG to pyruvate with the generation of ATP.
There is a delicate balance between the need to generate ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
to support energy requirements for cell metabolism and the need to maintain appropriate oxygenation/deoxygenation status of hemoglobin. This balance is maintained by dephosphorilation of 1,3-BPG to 2,3-BPG, which enhances the deoxygenation of hemoglobin. Low pH inhibits the activity of biphosphoglyceromutase and activates bisphosphoglyerate phosphatase, which favors generation of ATP.
Effects of binding

Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
and positive polarity by forming salt bridges with lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
and histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues in the four subunits of hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
. The R state, with oxygen bound to a heme group, has a different conformation and does not allow this interaction. By itself, hemoglobin has sigmoid-like kinetics, which makes easier another subunits’ binding (the first molecule of oxygen helps the following to link).
By selectively binding to deoxyhemoglobin, 2,3-BPG stabilizes the T state conformation, making it harder for oxygen to bind hemoglobin and more likely to be released to adjacent tissues. 2,3-BPG is part of a feedback loop that can help prevent tissue hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
in conditions where it is most likely to occur. Conditions of low tissue oxygen concentration such as high altitude (2,3-BPG levels are higher in those acclimated to high altitudes), airway obstruction
Airway obstruction
Airway obstruction is a respiratory problem caused by increased resistance in the bronchioles that reduces the amount of air inhaled in each breath and the oxygen that reaches the pulmonary arteries...
, or congestive heart failure
Congestive heart failure
Heart failure often called congestive heart failure is generally defined as the inability of the heart to supply sufficient blood flow to meet the needs of the body. Heart failure can cause a number of symptoms including shortness of breath, leg swelling, and exercise intolerance. The condition...
will tend to cause RBCs to generate more 2,3-BPG in their effort to generate energy by allowing more oxygen to be released in tissues deprived of oxygen. Ultimately, this mechanism increases oxygen release from RBCs under circumstances where it is needed most. This release is potentiated by the Bohr effect
Bohr effect
Bohr effect is a property of hemoglobin first described in 1904 by the Danish physiologist Christian Bohr , which states that an increasing concentration of protons and/or carbon dioxide will reduce the oxygen affinity of hemoglobin...
in tissues with high energetic demands. Bohr effect is another useful way to solve the affinity problem of the hemoglobin, and it’s related to the pH and the CO2.
It’s important to highlight that the behaviour of myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
doesn’t work in the same way, as 2,3-BPG has no effect on it.
Fetal hemoglobin
It is interesting to note that fetal hemoglobinFetal hemoglobin
Fetal hemoglobin, or foetal haemoglobin, is the main oxygen transport protein in the fetus during the last seven months of development in the uterus and in the newborn until roughly 6 months old...
(HbF) exhibits a low affinity for 2,3-BPG, resulting in a higher binding affinity for oxygen. This increased oxygen-binding affinity relative to that of adult hemoglobin (HbA) is due to HbF's having two α/γ dimers as opposed to the two α/β dimers of HbA. The positive histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues of HbA β-subunits that are essential for forming the 2,3-BPG binding pocket are replaced by serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
residues in HbF γ-subunits. Like that, histidine nº143 gets lost, so 2,3-BPG has difficulties in linking to the fetal hemoglobin, and it looks like the pure hemoglobin.
That’s the way O2 flows from the mother to the fetus.
As we can see in the following image, fetal hemoglobin has more affinity to oxygen than adult hemoglobin. Moreover, myoglobin has the highest affinity to oxygen.

Differences between myoglobin (Mb), fetal hemoglobin (Hb F), adult hemoglobin (Hb A)
Diseases related to 2,3-BPG
- Hyperthyroidism
A 2004 study checked the effects of thyroid hormone on 2,3-BPG levels. The result was that the hyperthyroidism modulates in vivo 2,3-BPG content in erythrocytes by changes in the expression of phosphoglycerate mutase
Phosphoglycerate mutase
-Overview:Phosphoglycerate mutase is an enzyme that catalyzes step 8 of glycolysis. It catalyzes the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate to 2-phosphoglycerate through a 2,3-bisphosphoglycerate intermediate.This enzyme is...
(PGM) and 2,3-BPG synthase.
This result shows that the increase in the 2,3-BPG content of erythrocytes observed in hyperthyroidism doesn’t depend on any variation in the rate of circulating hemoglobin, but seems to be a direct consequence of the stimulating effect of thyroid hormones on erythrocyte glycolytic activity.
- Iron deficiency anaemia
This illness is characterized by a lack of iron, and as 2,3-BPG needs this chemical element to be synthesized, BPG concentration decreases and hemoglobin binds tightly to oxygen. As a result, oxygen release to tissue is reduced.
- Chronic respiratory disease with hypoxia Hypoxia (medical)Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
Recently, scientists have found similarities between low amounts of 2,3-BPG with the occurrence of high altitude pulmonary edema at high altitudes.
| n | Hb (g/dl) | 2,3-BPG (mM) | ||
|---|---|---|---|---|
| 1 | Normality | 120 | 14.2 ± 1.6 | 4.54 ± 0.57 |
| 2 | Hyperthyroidism | 35 | 13.7 ± 1.4 | 5.66 ± 0.69 |
| 3 | Iron deficiency anaemia | 40 | 10.0 ± 1.7 | 5.79 ± 1.02 |
| 4 | Chronic respiratory disease with hypoxia | 47 | 16.4 ± 2.2 | 5.29 ± 1.13 |

