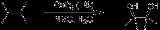
Upjohn dihydroxylation
Encyclopedia
Upjohn dihydroxylation is an organic reaction
converting an alkene
to a cis vicinal
diol
, and was developed by V. VanRheenen, R. C. Kelly and D. Y. Cha of the Upjohn Company, USA in 1976. It is a catalytic system using N-Methylmorpholine N-oxide
(NMO) as stoichiometric re-oxidant for the Osmium tetroxide, and is superior to previous catalytic methods.

Prior to this method, use of stoichiometric amounts of the toxic and expensive osmium tetroxide was often necessary. The Upjohn dihydroxylation is still often used for the formation of cis-vicinal diols, however it can be slow and is prone to over-oxidation of the substrate to the vicinal di-ketone. One of the peculiarities of the dihydroxylation of olefins is that the standard "racemic" method (the Upjohn dihydroxyation) is slower and often lower yielding than the asymmetric method (the Sharpless asymmetric dihydroxylation
).
, replacing the chiral ligands with the achiral quinuclidine
to give a racemic reaction product (assuming an achiral starting material is employed). This approach takes advantage of the fact that when using the Sharpless alkaloid ligands, the dihydroxylation of alkenes is faster and higher yielding than in their absence. This phenomenon became known as "ligand accelerated catalysis" a term coined by Barry Sharpless during the development of the asymmetric reaction.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
converting an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to a cis vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
, and was developed by V. VanRheenen, R. C. Kelly and D. Y. Cha of the Upjohn Company, USA in 1976. It is a catalytic system using N-Methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
(NMO) as stoichiometric re-oxidant for the Osmium tetroxide, and is superior to previous catalytic methods.

Prior to this method, use of stoichiometric amounts of the toxic and expensive osmium tetroxide was often necessary. The Upjohn dihydroxylation is still often used for the formation of cis-vicinal diols, however it can be slow and is prone to over-oxidation of the substrate to the vicinal di-ketone. One of the peculiarities of the dihydroxylation of olefins is that the standard "racemic" method (the Upjohn dihydroxyation) is slower and often lower yielding than the asymmetric method (the Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
).
Improvements to Upjohn Dihydroxylation
In response to these problems, Stuart Warren and co-workers employed identical reaction conditions to the Sharpless asymmetric dihydroxylationSharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
, replacing the chiral ligands with the achiral quinuclidine
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKa of the conjugate acid of 11.0. This is due to greater availability of the nitrogen lone pair...
to give a racemic reaction product (assuming an achiral starting material is employed). This approach takes advantage of the fact that when using the Sharpless alkaloid ligands, the dihydroxylation of alkenes is faster and higher yielding than in their absence. This phenomenon became known as "ligand accelerated catalysis" a term coined by Barry Sharpless during the development of the asymmetric reaction.
See also
- Milas hydroxylationMilas hydroxylationThe Milas hydroxylation is an organic reaction converting an alkene to a vicinal diol, and was developed by N. A. Milas in the 1930s. The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium, vanadium, or chromium oxide.The reaction...
- Sharpless asymmetric dihydroxylationSharpless asymmetric dihydroxylationSharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....

