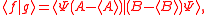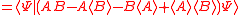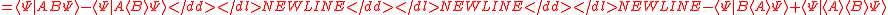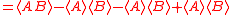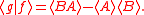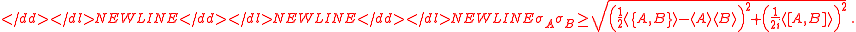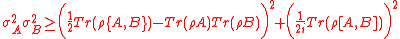Uncertainty principle derivations
Encyclopedia
There have been several mathematical improvements on the Heisenberg uncertainty principle
. Since the time of the original formulation, the uncertainty relation has become increasingly nuanced; our understanding of it has undergone both a qualitative and a quantitative change. On the qualitative side, uncertainty is seen to have a broader scope than the limited version of Heisenberg's Gedankenexperiment
; it is seen in terms of the multifaceted process of quantum measurement
. On the quantitative side, Heisenberg's uncertainty principle has been translated into the more formal language of quantum mechanics and given various interpretations and reformulations.
The Heisenberg uncertainty relation and its more formal versions deal explicitly with the quantum operators for position, and for momentum,
and for momentum, 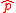 . Both the Robertson and Schrödinger uncertainty relations were derived for generalized quantum operators. Yet other formulations have moved beyond the framework which inspired these traditional uncertainty relations.
. Both the Robertson and Schrödinger uncertainty relations were derived for generalized quantum operators. Yet other formulations have moved beyond the framework which inspired these traditional uncertainty relations.
where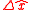 is the uncertainty of knowledge about the position of the particle,
is the uncertainty of knowledge about the position of the particle, 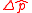 is the uncertainty associated with the momentum of the particle, and
is the uncertainty associated with the momentum of the particle, and 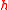 is the Planck constant
is the Planck constant
. Max Planck had introduced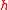 to describe the smallest possible amount of "action" (energy multiplied by time) involved in quantum processes and Heisenberg used this as his lower bound.
to describe the smallest possible amount of "action" (energy multiplied by time) involved in quantum processes and Heisenberg used this as his lower bound.
After Heisenberg formulated his equation, Kennard and later Weyl derived the more formal relation
where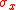 and
and 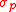 are standard deviations of the two operators
are standard deviations of the two operators  and
and 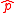 defined for an arbitrary Hermitian operator
defined for an arbitrary Hermitian operator  as
as
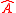 and
and 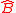
where and
and 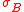 are the standard deviations and where
are the standard deviations and where 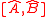 represents the commutator
represents the commutator  or the incompatibility of the two operators
or the incompatibility of the two operators 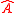 and
and  . The majority of quantum textbooks rederive the Robertson uncertainty relation when presenting a generalized derivation of the uncertainty principle (see, for example,).
. The majority of quantum textbooks rederive the Robertson uncertainty relation when presenting a generalized derivation of the uncertainty principle (see, for example,).
Schrödinger derived the following uncertainty relation
The difference between Schrödinger's and Robertson's version is the first squared term under the square root, known in a classical statistics sense as the covariance, consisting of the anti-commutator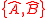 , defined as
, defined as 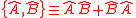 , and the product of two expectation values
, and the product of two expectation values 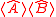 . As pointed out originally by Schrödinger, the Schrödinger uncertainty relation often has the effect of raising the lower bound on uncertainty. Because the added terms are squared, the Schrödinger additions are either positive or zero. This will obviously either increase the uncertainty by some amount, or make no difference.
. As pointed out originally by Schrödinger, the Schrödinger uncertainty relation often has the effect of raising the lower bound on uncertainty. Because the added terms are squared, the Schrödinger additions are either positive or zero. This will obviously either increase the uncertainty by some amount, or make no difference.
It can also be shown that the Schrödinger uncertainty relation for mixed states
is given by
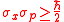 in which he sought (at the suggestion of Wolfgang Pauli) to relate the two standard deviations
in which he sought (at the suggestion of Wolfgang Pauli) to relate the two standard deviations  and
and 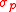 . The Schwarz inequality was also used by Robertson in his 1929 derivation and Schrödinger in his 1930 derivation, who both cite Weyl.
. The Schwarz inequality was also used by Robertson in his 1929 derivation and Schrödinger in his 1930 derivation, who both cite Weyl.
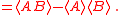
Schrödinger's method of derivation differed from that of Robertson. The critical step that differentiates the two is Schrödinger's use of the fact that the product of two Hermitian operators is not in general Hermitian, but can be split into a "symmetric product and half its commutator":
Schrödinger then comments that this splitting corresponds to a splitting of a complex number into real and imaginary parts, and thus an expectation value can also be split into real and imaginary parts. This corresponds directly to Eq. (3) from the boxed derivations above. If one takes only the imaginary part, one recovers the Robertson relation; if one takes both the real and imaginary parts, the Schrödinger relation results.
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
. Since the time of the original formulation, the uncertainty relation has become increasingly nuanced; our understanding of it has undergone both a qualitative and a quantitative change. On the qualitative side, uncertainty is seen to have a broader scope than the limited version of Heisenberg's Gedankenexperiment
Heisenberg's microscope
Heisenberg's microscope exists only as a thought experiment, one that was proposed by Werner Heisenberg, criticized by his mentor Niels Bohr, and subsequently served as the nucleus of some commonly held ideas, and misunderstandings, about Quantum Mechanics...
; it is seen in terms of the multifaceted process of quantum measurement
Measurement in quantum mechanics
The framework of quantum mechanics requires a careful definition of measurement. The issue of measurement lies at the heart of the problem of the interpretation of quantum mechanics, for which there is currently no consensus....
. On the quantitative side, Heisenberg's uncertainty principle has been translated into the more formal language of quantum mechanics and given various interpretations and reformulations.
The Heisenberg uncertainty relation and its more formal versions deal explicitly with the quantum operators for position,
 and for momentum,
and for momentum, 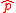 . Both the Robertson and Schrödinger uncertainty relations were derived for generalized quantum operators. Yet other formulations have moved beyond the framework which inspired these traditional uncertainty relations.
. Both the Robertson and Schrödinger uncertainty relations were derived for generalized quantum operators. Yet other formulations have moved beyond the framework which inspired these traditional uncertainty relations.The Heisenberg Uncertainty Relation
Heisenberg originally thought of uncertainty in an intuitive way. He pictured a microscope through which one could view very small objects. Heisenberg proposed a Gedankenexperiment in which one uses high-frequency photons to improve the resolution of what is being imaged. In his seminal paper on uncertainty Heisenberg explains, "Let one illuminate the electron and observe it under a microscope. Then the highest attainable accuracy in the measurement of position is governed by the wavelength of the light. However, in principle one can build, say, a gamma-ray microscope and with it carry out the determination of position with as much accuracy as one wants." However, he notes, since gamma rays have high-frequency and energy, they will greatly disturb the electron being observed. The observed electron undergoes "a discontinuous change of momentum." Essentially, it is a Catch-22; the high-frequency gamma ray determines the position very accurately, yet it disturbs the electron so much that one loses track of the momentum. Likewise, if one uses lower frequency photons, such as microwaves, the momentum will be much more certain, but the position will become fuzzy. All of this is encapsulated in his uncertainty principlewhere
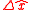 is the uncertainty of knowledge about the position of the particle,
is the uncertainty of knowledge about the position of the particle, 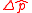 is the uncertainty associated with the momentum of the particle, and
is the uncertainty associated with the momentum of the particle, and 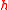 is the Planck constant
is the Planck constantPlanck constant
The Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
. Max Planck had introduced
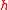 to describe the smallest possible amount of "action" (energy multiplied by time) involved in quantum processes and Heisenberg used this as his lower bound.
to describe the smallest possible amount of "action" (energy multiplied by time) involved in quantum processes and Heisenberg used this as his lower bound.After Heisenberg formulated his equation, Kennard and later Weyl derived the more formal relation
where
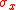 and
and 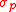 are standard deviations of the two operators
are standard deviations of the two operators  and
and 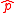 defined for an arbitrary Hermitian operator
defined for an arbitrary Hermitian operator  as
as
-
-
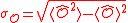 .
.
-
The Robertson Uncertainty Relation
Going even further, Robertson generalized Weyl's equation for arbitrary Hermitian operators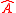 and
and 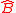
where
 and
and 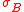 are the standard deviations and where
are the standard deviations and where 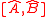 represents the commutator
represents the commutator  or the incompatibility of the two operators
or the incompatibility of the two operators 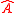 and
and  . The majority of quantum textbooks rederive the Robertson uncertainty relation when presenting a generalized derivation of the uncertainty principle (see, for example,).
. The majority of quantum textbooks rederive the Robertson uncertainty relation when presenting a generalized derivation of the uncertainty principle (see, for example,).| Derivation of the Robertson uncertainty relation |
|---|
(dropping the operator hat  notation), based upon the standard deviation definition, we have notation), based upon the standard deviation definition, we havewe let 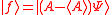 and thus and thusSimilarly, for any other Hermitian operator  in the same state in the same statefor 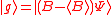 . .The product of the two deviations can thus be expressed as In order to relate the two vectors  and and 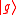 , we use the Schwarz inequality which is defined as , we use the Schwarz inequality which is defined as
and thus Eq. can be written as Since 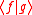 is in general a complex number, we use the fact that the modulus squared of any complex number is in general a complex number, we use the fact that the modulus squared of any complex number  is defined as is defined as 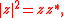 where where  is the complex conjugate of is the complex conjugate of  . The modulus squared can also be expressed as . The modulus squared can also be expressed as
we let 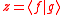 and and 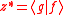 and substitute these into the equation above to get and substitute these into the equation above to get
The inner product 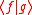 is written out explicitly as is written out explicitly as
and using the fact that  and and  are Hermitian operators, we find are Hermitian operators, we find
|
The Schrödinger Uncertainty Relation
Though Heisenberg's uncertainty principle and Robertson's version of the uncertainty relation are part of the foundation of quantum mechanics, other versions of the uncertainty relation-including the Schrödinger uncertainty relation-have received comparatively little attention in the physics literature. The Schrödinger uncertainty relation was initially published in an inconspicuous German journal that periodically reported on the activity of the Prussian Academy. This may have led to the cursory coverage the Schrödinger uncertainty relation receives in the literature.Schrödinger derived the following uncertainty relation
The difference between Schrödinger's and Robertson's version is the first squared term under the square root, known in a classical statistics sense as the covariance, consisting of the anti-commutator
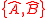 , defined as
, defined as 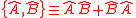 , and the product of two expectation values
, and the product of two expectation values 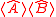 . As pointed out originally by Schrödinger, the Schrödinger uncertainty relation often has the effect of raising the lower bound on uncertainty. Because the added terms are squared, the Schrödinger additions are either positive or zero. This will obviously either increase the uncertainty by some amount, or make no difference.
. As pointed out originally by Schrödinger, the Schrödinger uncertainty relation often has the effect of raising the lower bound on uncertainty. Because the added terms are squared, the Schrödinger additions are either positive or zero. This will obviously either increase the uncertainty by some amount, or make no difference.| Derivation of the Schrödinger uncertainty relation |
|---|
(dropping the operator hat  notation), based upon the standard deviation definition, we have notation), based upon the standard deviation definition, we havewe let 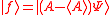 and thus and thusSimilarly, for any other Hermitian operator  in the same state in the same statefor 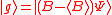 . .The product of the two deviations can thus be expressed as In order to relate the two vectors 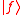 and and 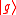 , we use the Schwarz inequality which is defined as , we use the Schwarz inequality which is defined as
and thus Eq. can be written as Since 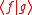 is in general a complex number, we use the fact that the modulus squared of any complex number is in general a complex number, we use the fact that the modulus squared of any complex number  is defined as is defined as 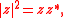 where where  is the complex conjugate of is the complex conjugate of  . The modulus squared can also be expressed as . The modulus squared can also be expressed aswe let 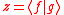 and and 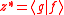 and substitute these into the equation above to get and substitute these into the equation above to get
The inner product 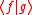 is written out explicitly as is written out explicitly as
and using the fact that  and and  are Hermitian operators, we find are Hermitian operators, we find
|
It can also be shown that the Schrödinger uncertainty relation for mixed states
Density matrix
In quantum mechanics, a density matrix is a self-adjoint positive-semidefinite matrix of trace one, that describes the statistical state of a quantum system...
is given by
Discussion of the Schrödinger Uncertainty Relation
An analysis of the derivations of uncertainty reveals that uncertainty first comes into play with the Schwarz inequality and the related inequality for mixed states. In the context of the uncertainty principle, the Schwarz inequality was used first by Weyl in his 1928 derivation of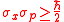 in which he sought (at the suggestion of Wolfgang Pauli) to relate the two standard deviations
in which he sought (at the suggestion of Wolfgang Pauli) to relate the two standard deviations  and
and 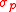 . The Schwarz inequality was also used by Robertson in his 1929 derivation and Schrödinger in his 1930 derivation, who both cite Weyl.
. The Schwarz inequality was also used by Robertson in his 1929 derivation and Schrödinger in his 1930 derivation, who both cite Weyl.Schrödinger's method of derivation differed from that of Robertson. The critical step that differentiates the two is Schrödinger's use of the fact that the product of two Hermitian operators is not in general Hermitian, but can be split into a "symmetric product and half its commutator":
Schrödinger then comments that this splitting corresponds to a splitting of a complex number into real and imaginary parts, and thus an expectation value can also be split into real and imaginary parts. This corresponds directly to Eq. (3) from the boxed derivations above. If one takes only the imaginary part, one recovers the Robertson relation; if one takes both the real and imaginary parts, the Schrödinger relation results.


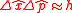
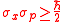
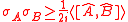
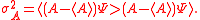

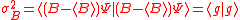
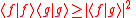 ,
,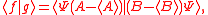
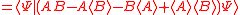
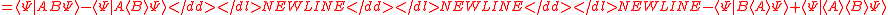
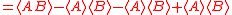
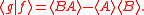
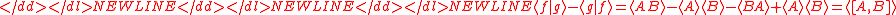

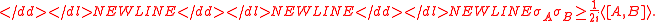

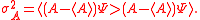
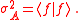
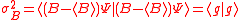
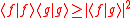 ,
,