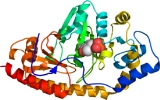
Tyrosine aminotransferase
Encyclopedia
Tyrosine aminotransferase (or tyrosine transaminase) is an enzyme present in the liver and catalyzes the conversion of tyrosine
to 4-hydroxyphenylpyruvate. In humans, the tyrosine aminotransferase protein is encoded by the TAT gene
. A deficiency of the enzyme in humans can result in what is known as Type II Tyrosinemia, wherein there is an abundance of tyrosine as a result of tyrosine failing to undergo an aminotransferase reaction to form 4-hydroxyphenylpyruvate.
, the prosthetic group pyridoxal phosphate, and the resulting product 4-hydroxyphenylpyruvate
.
 Each side of the dimer protein includes pyridoxal pyruvate (PLP) bonded to the Lys280 residue of the tyrosine aminotransferase molecule. The amine group of tyrosine attacks the alpha carbon of the imine bonded to Lys280, forming a tetrahedral complex and then kicking off the LYS-ENZ. This process is known as transimination by the act of switching out the imine group bonded to PLP. The newly formed PLP-TYR molecule is then attacked by a base.
Each side of the dimer protein includes pyridoxal pyruvate (PLP) bonded to the Lys280 residue of the tyrosine aminotransferase molecule. The amine group of tyrosine attacks the alpha carbon of the imine bonded to Lys280, forming a tetrahedral complex and then kicking off the LYS-ENZ. This process is known as transimination by the act of switching out the imine group bonded to PLP. The newly formed PLP-TYR molecule is then attacked by a base.
 A possible candidate for the base in the mechanism could be Lys280 that was just pushed off of PLP, which sequesters the newly formed amino group of the PLP-TYR molecule. In a similar mechanism of aspartate transaminase
A possible candidate for the base in the mechanism could be Lys280 that was just pushed off of PLP, which sequesters the newly formed amino group of the PLP-TYR molecule. In a similar mechanism of aspartate transaminase
, the lysine that forms the initial imine to PLP later acts as the base that attacks the tyrosine in transimination. The electrons left behind from the loss of the proton move down to form a new double bond to the imine, which in turn pushes the already double bonded electrons through PLP and end up as a lone pair on the positively charged nitrogen in the six-membered ring of the molecule. Water attacks the alpha carbon of the imine of PLP-TYR and through acyl substitution kicks off the nitrogen of PLP and forming pyridoxamine phosphate (PMP) and 4-hydroxyphenylpyruvate.
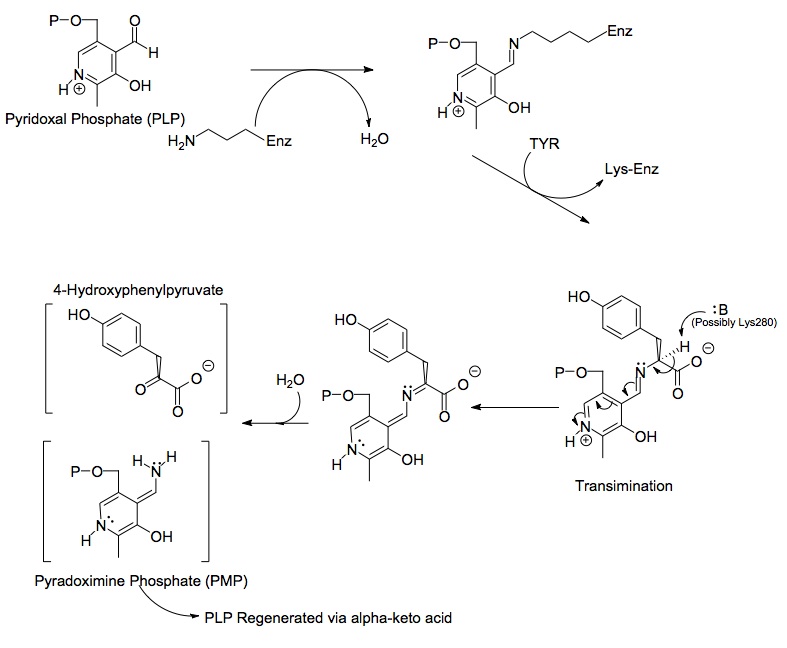
PMP is then regenerated into PLP by transferring its amine group to alpha-ketoglutarate, reforming it's aldehyde functional group. This is followed by another substitution reaction with the Lys280 residue to reform its imine linkage to the enzyme, forming ENZ-PLP.
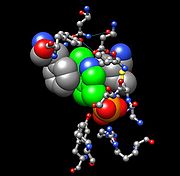 Tyrosine Aminotransferase as a dimer has two identical active sights. Lys280 is attached to PLP, which is held in place via two nonpolar amino acid side chains; phenylalanine
Tyrosine Aminotransferase as a dimer has two identical active sights. Lys280 is attached to PLP, which is held in place via two nonpolar amino acid side chains; phenylalanine
and isoleucine
(see thumbnail on right). The PLP is also held in place by hydrogen bonding to surrounding molecules mainly by its phosphate group.
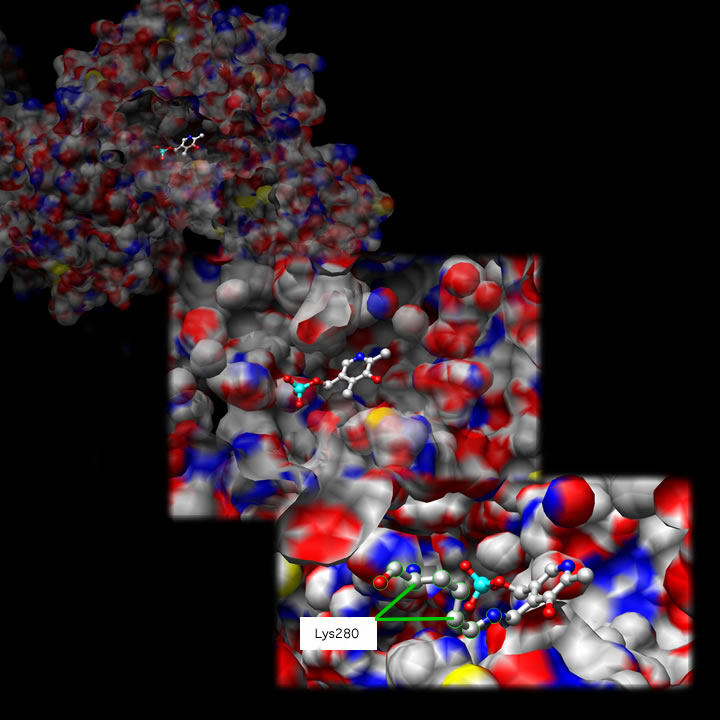
Shown below is one active site at three different magnifications:
is the most common metabolic disease associated with tyrosine aminotransferase. The disease results from a deficiency in hepatic tyrosine aminotransferase. Tyrosinemia type II (Richner-Hanhart syndrome, RHS) is a disease of autosomal recessive inheritance characterized by keratitis, palmoplantar hyperkeratosis, mental retardation, and elevated blood tyrosine levels. Keratitis in Tyrosinemia type II patients is caused by the deposition of tyrosine crystals in the cornea and results in corneal inflammation. The TAT gene is located on human chromosome 16q22-24 and extends over 10.9 kilobases (kb) containing 12 exons, and its 3.0 kb mRNA codes for a 454-amino acid protein of 50.4 kDa. Twelve different TAT gene mutations have been reported.
Christopher Mahnken, DePauw University 2009
(This page was a stub with limited information prior to our extensive expansion.)
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
to 4-hydroxyphenylpyruvate. In humans, the tyrosine aminotransferase protein is encoded by the TAT gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
. A deficiency of the enzyme in humans can result in what is known as Type II Tyrosinemia, wherein there is an abundance of tyrosine as a result of tyrosine failing to undergo an aminotransferase reaction to form 4-hydroxyphenylpyruvate.
Mechanism
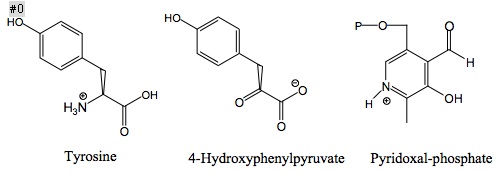
Structures of the three main molecules involved in chemical reaction catalyzed by the tyrosine aminotransferase enzyme are shown below: the amino acid tyrosineTyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, the prosthetic group pyridoxal phosphate, and the resulting product 4-hydroxyphenylpyruvate
4-Hydroxyphenylpyruvic acid
4-Hydroxyphenylpyruvic acid is an intermediate in the metabolism of the amino acid phenylalanine. The aromatic side chain of phenylalanine is hydroxylated by the enzyme phenylalanine hydroxylase to form tyrosine. The conversion from tyrosine to 4-HPPA is in turn catalyzed by tyrosine...
.



Aspartate transaminase
Aspartate transaminase , also called aspartate aminotransferase or serum glutamic oxaloacetic transaminase , is a pyridoxal phosphate -dependent transaminase enzyme . AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in...
, the lysine that forms the initial imine to PLP later acts as the base that attacks the tyrosine in transimination. The electrons left behind from the loss of the proton move down to form a new double bond to the imine, which in turn pushes the already double bonded electrons through PLP and end up as a lone pair on the positively charged nitrogen in the six-membered ring of the molecule. Water attacks the alpha carbon of the imine of PLP-TYR and through acyl substitution kicks off the nitrogen of PLP and forming pyridoxamine phosphate (PMP) and 4-hydroxyphenylpyruvate.

PMP is then regenerated into PLP by transferring its amine group to alpha-ketoglutarate, reforming it's aldehyde functional group. This is followed by another substitution reaction with the Lys280 residue to reform its imine linkage to the enzyme, forming ENZ-PLP.
Active Site

Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
and isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
(see thumbnail on right). The PLP is also held in place by hydrogen bonding to surrounding molecules mainly by its phosphate group.
Shown below is one active site at three different magnifications:

Pathology
TyrosinemiaTyrosinemia
Tyrosinemia is an error of metabolism, usually inborn, in which the body cannot effectively break down the amino acid tyrosine. Symptoms include liver and kidney disturbances and mental retardation...
is the most common metabolic disease associated with tyrosine aminotransferase. The disease results from a deficiency in hepatic tyrosine aminotransferase. Tyrosinemia type II (Richner-Hanhart syndrome, RHS) is a disease of autosomal recessive inheritance characterized by keratitis, palmoplantar hyperkeratosis, mental retardation, and elevated blood tyrosine levels. Keratitis in Tyrosinemia type II patients is caused by the deposition of tyrosine crystals in the cornea and results in corneal inflammation. The TAT gene is located on human chromosome 16q22-24 and extends over 10.9 kilobases (kb) containing 12 exons, and its 3.0 kb mRNA codes for a 454-amino acid protein of 50.4 kDa. Twelve different TAT gene mutations have been reported.
Authors
Christopher Cadle, DePauw University 2010Christopher Mahnken, DePauw University 2009
(This page was a stub with limited information prior to our extensive expansion.)

