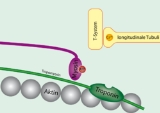
Tropomyosin
Encyclopedia

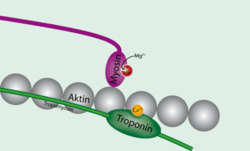
Troponin
400px|thumb|right|alt = Colored dice with checkered background|Ribbon representation of the human cardiac troponin core complex in the calcium-saturated form...
complex, associate with actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
in muscle fibers and regulate muscle contraction by regulating the binding of myosin
Myosin
Myosins comprise a family of ATP-dependent motor proteins and are best known for their role in muscle contraction and their involvement in a wide range of other eukaryotic motility processes. They are responsible for actin-based motility. The term was originally used to describe a group of similar...
. In resting muscle, tropomyosin overlays the myosin binding sites on actin, with a single tropomyosin molecule spanning 7 actin subunits, and is "locked" down in this position by troponin T
Troponin T
Troponin T is a part of the troponin complex. It binds to tropomyosin, interlocking them to form a troponin-tropomyosin complex.The tissue specific subtypes are:* Slow skeletal troponin T1, TNNT1 * Cardiac troponin T2, TNNT2...
(tropomyosin binding troponin) and troponin I (inhibitory troponin). Upon release of calcium from the sarcoplasmic reticulum calcium binds to troponin C (calcium binding troponin). This "unlocks" tropomyosin from actin, allowing it to move away from the binding groove. Myosin heads can now access the binding sites on actin. Once one myosin head binds, this fully displaces tropomyosin and allows additional myosin heads to bind, initiating muscle shortening and contraction. Once calcium is pumped out of the cytoplasm and calcium levels return to normal, tropomyosin again binds to actin, preventing myosin from binding.
Sliding filament theory
Tropomyosin is an alpha helical coiled coilCoiled coil
A coiled coil is a structural motif in proteins, in which 2-7 alpha-helices are coiled together like the strands of a rope . Many coiled coil type proteins are involved in important biological functions such as the regulation of gene expression e.g. transcription factors...
protein dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
that binds end to end along F actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
filaments in striated muscle
Striated muscle
Striated muscle tissue is a form of fibers that are combined into parallel fibers. More specifically, it can refer to:* Cardiac muscle .* Skeletal muscle* Branchiomeric muscles...
. Tropomyosin blocks myosin
Myosin
Myosins comprise a family of ATP-dependent motor proteins and are best known for their role in muscle contraction and their involvement in a wide range of other eukaryotic motility processes. They are responsible for actin-based motility. The term was originally used to describe a group of similar...
binding and hence crossbridge cycling in the absence of Ca2+. Ca2+ influx from the sarcoplasmic reticulum of striated muscle myocytes binds to troponin and subsequently moves tropomyosin on the F-actin filament, exposing the myosin binding sites.
Two-State Model
In this model, tropomyosin can take two conformations: blocked or open. In the blocked state, tropomysoin is locked down to the actin via the troponin complex and myosin is unable to bind actin. Upon release of Ca2+, the troponin complex—specifically troponin C (TnC)—binds calcium, resulting in a conformational change of tropomyosin that physically shifts the protein on actin and fully exposes the myosin binding sites. Myosin can now bind and perform work.Three-State Model
In this model, tropomyosin can take three conformations—blocked, closed, and open. The blocked state is the same as the two-state model. Upon release of Ca2+, however, the troponin complex simply unlocks the tropomyosin from actin, but does not physically move the molecule. The tropomyosin is now free to fluctuate across the actin filament, sometimes blocking it and sometimes exposing it. On average, this can be modeled as a partially blocked myosin binding site and is known as the closed state. If a myosin is able to sneak in and bind actin, it now sterically hinders tropomyosin from covering the myosin binding site, completely exposing this site as well as adjacent myosin binding sites. This is the open state.Recent structural visualization and kinetic modeling has provided evidence for this model; however, this three-state model is still debated by experts who believe that two-state regulation of muscle contraction (involving a blocked and open state) is sufficient to explain current experimental data and models.
Allergies
Tropomyosin is a pan-allergen (an allergen widely distributed in nature) because it is a highly conserved protein among species. Certain tropomyosins are known to cause allergies in certain people, and those who have cross-reactive allergies can get symptoms from a range of sources due to a common allergen found in all these sources: ShrimpShrimp
Shrimp are swimming, decapod crustaceans classified in the infraorder Caridea, found widely around the world in both fresh and salt water. Adult shrimp are filter feeding benthic animals living close to the bottom. They can live in schools and can swim rapidly backwards. Shrimp are an important...
, dust mites and mollusks. This common allergen is the reason why some people sensitized with mite tropomyosin could have an allergic reaction after eating seafood.
Genes

- TPM1TPM1Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene.-Further reading:- External links :*...
- TPM2TPM2Tropomyosin beta chain is a protein that in humans is encoded by the TPM2 gene.-Interactions:TPM2 has been shown to interact with RRAD, PDLIM7 and TPM1.-External links:* -Further reading:...
- TPM3
- TPM4TPM4Tropomyosin alpha-4 chain is a protein that in humans is encoded by the TPM4 gene.-Further reading:...

