
Transient kinetic isotope fractionation
Encyclopedia
Transient kinetic isotope effects (or fractionation) occur when the reaction
leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation
equations or with steady-state kinetic fractionation
equations (also known as the Rayleigh equation). In these instances, the General Equations for Biochemical Isotope Kinetics (GEBIK) and the General Equations for Biochemical Isotope Fractionation (GEBIF) can be used.
The GEBIK and GEBIF equations are the most generalized approach to describe isotopic effects in any chemical
, catalytic reaction and biochemical reactions because they can describe isotopic effects in equilibrium reactions, kinetic chemical reactions and kinetic biochemical reactions. In the latter two cases, they can describe both stationary and non-stationary fractionation (i.e., variable and inverse fractionation). In general, isotopic effects depend on the number of reactants and on the number of combinations resulting from the number of substitutions in all reactants and products. Describing with accuracy isotopic effects, however, depends also on the specific rate law
used to describe the chemical or biochemical reaction that produces isotopic effects. Normally, regardless of whether a reaction is purely chemical or whether it involves some enzyme of biological nature, the equations used to describe isotopic effects base on first-order kinetics. This approach systematically leads to isotopic effects that can be described by means of the Rayleigh equation. In this case, isotopic effects will always be expressed as a constant, hence will not be able to describe isotopic effects in reactions where fractionation and enrichment are variable or inverse during the course of a reaction. Most chemical reactions do not follow first-order kinetics; neither biochemical reactions can normally be described with first-order kinetics. To properly describe isotopic effects in chemical or biochemical reactions, different approaches must be employed such as the use of Michaelis-Menten reaction order (for chemical reactions) or coupled Michaelis-Menten and Monod reaction orders (for biochemical reactions). However, conversely to Michaelis-Menten kinetics, GEBIK and GEBIF equations are solved under the hypothesis of non steady state. This characteristic allows GEBIK and GEBIF to capture transient isotopic effects.
 substrate concentration
substrate concentration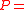 product concentration
product concentration enzyme concentration
enzyme concentration complex concentration
complex concentration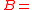 biomass concentration
biomass concentration
Both S and P contain at least one isotopic expression of a tracer atom. For instance, if the carbon element is used as a tracer, both S and P contain at least one C atom, which may appear as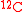 and
and 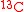 . The isotopic expression within a molecule is
. The isotopic expression within a molecule is
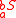
where is the number of tracer atoms within S, while
is the number of tracer atoms within S, while  is the number of isotopic substitutions in the same molecule. The condition
is the number of isotopic substitutions in the same molecule. The condition  must be satisfied. For example, the N
must be satisfied. For example, the N product in which 1 isotopic substitution occurs (e.g.,
product in which 1 isotopic substitution occurs (e.g., 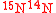 ) will be described by
) will be described by 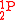 .
.
Substrates and products appear in a chemical reaction with specific stoichiometric coefficients. When chemical reactions comprise combinations of reactants and products with various isotopic expressions, the stoichiometric coefficients are functions of the isotope substitution number. If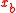 and
and  are the stoichiometric coefficient for
are the stoichiometric coefficient for 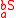 substrate and
substrate and 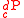 product, a reaction takes the form
product, a reaction takes the form
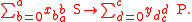
For example, in the reaction
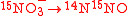 , the notation is
, the notation is 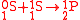 with
with 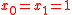 for both isotopologue reactants of the same substrate with substitution number
for both isotopologue reactants of the same substrate with substitution number  and
and  , and with
, and with 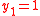 for
for 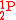 and
and 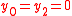 because the reaction does not comprise production of
because the reaction does not comprise production of 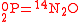 and
and 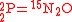 .
.
For isotopomers, the substitution location is taken into account as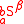 and
and 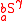 , where
, where 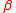 and
and  indicate a different expressions of the same isotopologue
indicate a different expressions of the same isotopologue 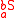 . Isotopomers only exist when
. Isotopomers only exist when  and
and  . The substitution location has to be specifically defined depending on the number of tracer atoms
. The substitution location has to be specifically defined depending on the number of tracer atoms  , number of substitutions
, number of substitutions 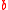 , and molecule structure. For multiatomic molecules that are symmetric with respect to tracer position, there is no need to specify the substitution position when
, and molecule structure. For multiatomic molecules that are symmetric with respect to tracer position, there is no need to specify the substitution position when  . For example, one substitution of deuterium D =
. For example, one substitution of deuterium D =  in the symmetric methane molecule CDH
in the symmetric methane molecule CDH does not require the use of the right superscript. In the case that
does not require the use of the right superscript. In the case that  , the substitution location has to be specified, while for CHD
, the substitution location has to be specified, while for CHD and CD
and CD it is not required. For example, two D substitutions in CD
it is not required. For example, two D substitutions in CD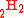 can occur in adjacent or non-adjacent locations. Using this notation, the reaction
can occur in adjacent or non-adjacent locations. Using this notation, the reaction  can be written as
can be written as

where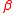 in
in 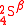 defines only one of the two methane forms (either with adjacent or non-adjacent D atoms). The location of D in the two isotopologue water molecules produced on the right-hand side of the reaction has not been indicated because D is present in only one water molecule at saturation, and because the water molecule is symmetric. For asymmetric and multiatomic molecules with
defines only one of the two methane forms (either with adjacent or non-adjacent D atoms). The location of D in the two isotopologue water molecules produced on the right-hand side of the reaction has not been indicated because D is present in only one water molecule at saturation, and because the water molecule is symmetric. For asymmetric and multiatomic molecules with  and
and  , definition of the substitution location is always required. For instance, the isotopomers of the (asymmetric) nitrous oxide molecule
, definition of the substitution location is always required. For instance, the isotopomers of the (asymmetric) nitrous oxide molecule 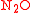 are
are 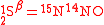 and
and 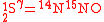 .
.
Reactions of asymmetric isotopomers can be written using the partitioning coefficient as
as

where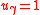 . For example, using N isotope tracers, the isotopomer reactions
. For example, using N isotope tracers, the isotopomer reactions
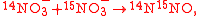
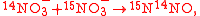
can be written as one reaction in which each isotopomer product is multiplied by its partition coefficient as
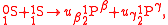
with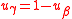 . More generally, the tracer element does not necessarily occur in only one substrate and one product. If
. More generally, the tracer element does not necessarily occur in only one substrate and one product. If 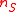 substrates react releasing
substrates react releasing 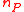 products, each having an isotopic expression of the tracer element, then the generilized reaction notation is
products, each having an isotopic expression of the tracer element, then the generilized reaction notation is
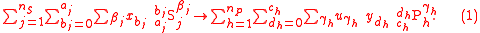
For instance, consider the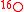 and
and 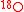 tracers in the reaction
tracers in the reaction
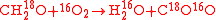
In this case the reaction can be written as

with two substrates and two products without indication of the substitution location because all molecules are symmetric.
Biochemical kinetic reactions of type (1) are often catalytic reactions in which one or more substrates,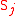 , bind to an enzyme, E, to form a reversible activated complex, C, which releases one or more
, bind to an enzyme, E, to form a reversible activated complex, C, which releases one or more
products,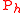 , and free, unchanged enzyme. These reactions belong to the type of reactions that can be described by Michaelis-Menten
, and free, unchanged enzyme. These reactions belong to the type of reactions that can be described by Michaelis-Menten
kinetics. Using this approach for substrate and product isotopologue and isotopomer expressions, and under the prescribed stoichiometric relationships among them, leads to the general reactions of the Michaelis-Menten type

with the index , where
, where  depends on the number of possible atomic combinations among all isotopologues and isotopomers. Here,
depends on the number of possible atomic combinations among all isotopologues and isotopomers. Here, 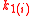 ,
,  , and
, and  are the rate constants indexed for each of the m reactions.
are the rate constants indexed for each of the m reactions.
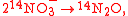
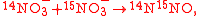
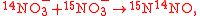
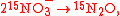
can be written as
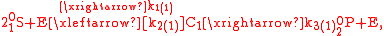


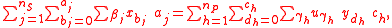

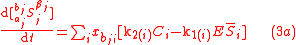
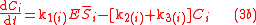
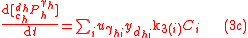
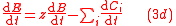
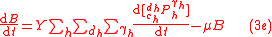 .
.
with , and where
, and where 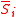 is the concentration of the most limiting substrate in each reaction i, z is the enzyme yield coefficient, Y is the yield coefficient expressing the biomass gain per unit of released product and
is the concentration of the most limiting substrate in each reaction i, z is the enzyme yield coefficient, Y is the yield coefficient expressing the biomass gain per unit of released product and  is the biomass mortality rate.
is the biomass mortality rate.
isotopologue
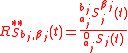
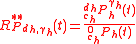
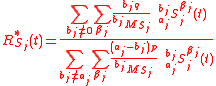
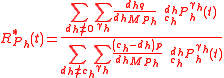
where ,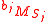 and
and 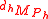 are the molecular weight of each isotopic expression of the substrate and product.
are the molecular weight of each isotopic expression of the substrate and product.
substrates and products
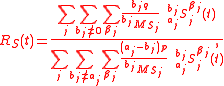
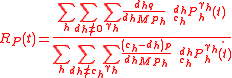
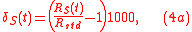
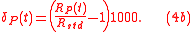
where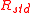 is a standard isotopic ration. Here, definition 3 of isotopic ratio has been used, however, any of the three definitions of isotopic ratio can equally be used.
is a standard isotopic ration. Here, definition 3 of isotopic ratio has been used, however, any of the three definitions of isotopic ratio can equally be used.
isotopic ratio

and the time-dependent fractionation factor
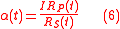
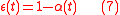
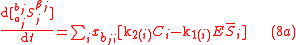
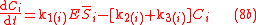
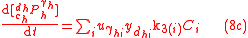
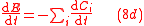
Eqs. (4) for isotopic compositions, Eq. (6) for the fractionation factor and Eq. (7) for the enrichment factor equally applies to the GEBIK equations under the BFEI hypothesis.
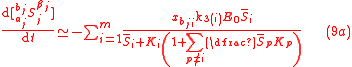
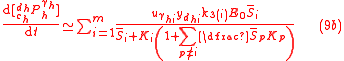
which are written in a form similar to the classical Micaelis-Menten equations for any substrate and product. Here, the equations also show that the various isotopologue and isotopomer substrates appear as competing species. Eqs. (4) for isotopic compositions, Eq. (6) for the fractionation factor and Eq. (7) for the enrichment factor equally applies to the GEBIK equations under the BFEI and QSS hypothesis.
 O consumption into N
O consumption into N according to the simultaneous set of reactions
according to the simultaneous set of reactions
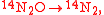
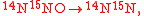
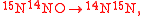
These can be rewritten using the notation introduced before as.
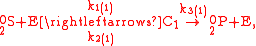
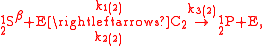
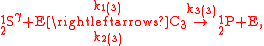
The substrate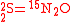 has not been included due to its scarcity. In addition, we have not specified the isotopic substitution in the N
has not been included due to its scarcity. In addition, we have not specified the isotopic substitution in the N product of the second and third reactions because N
product of the second and third reactions because N is symmetric. Assuming that the second and third reactions have identical reaction rates
is symmetric. Assuming that the second and third reactions have identical reaction rates  ,
,  , and
, and  , the full GEBIK and GEBIF equations are
, the full GEBIK and GEBIF equations are
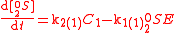
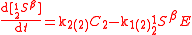
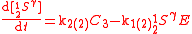
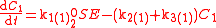
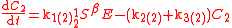
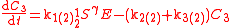
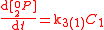

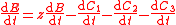
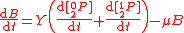
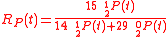

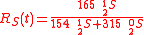
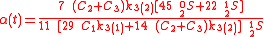
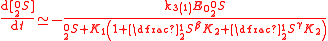

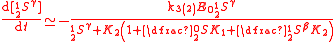

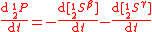
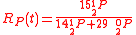
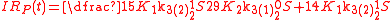
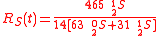
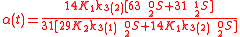
where K has been substituted with K
has been substituted with K because the rate
because the rate
constants in the third reaction have been assumed to equal those
of the second reaction.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
leading to isotope fractionation does not follow pure first-order kinetics and therefore isotopic effects cannot be described with the classical equilibrium fractionation
Equilibrium fractionation
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and forms the basis of the most widely used isotopic paleothermometers : D/H and 18O/16O records from ice...
equations or with steady-state kinetic fractionation
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
equations (also known as the Rayleigh equation). In these instances, the General Equations for Biochemical Isotope Kinetics (GEBIK) and the General Equations for Biochemical Isotope Fractionation (GEBIF) can be used.
The GEBIK and GEBIF equations are the most generalized approach to describe isotopic effects in any chemical
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, catalytic reaction and biochemical reactions because they can describe isotopic effects in equilibrium reactions, kinetic chemical reactions and kinetic biochemical reactions. In the latter two cases, they can describe both stationary and non-stationary fractionation (i.e., variable and inverse fractionation). In general, isotopic effects depend on the number of reactants and on the number of combinations resulting from the number of substitutions in all reactants and products. Describing with accuracy isotopic effects, however, depends also on the specific rate law
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
used to describe the chemical or biochemical reaction that produces isotopic effects. Normally, regardless of whether a reaction is purely chemical or whether it involves some enzyme of biological nature, the equations used to describe isotopic effects base on first-order kinetics. This approach systematically leads to isotopic effects that can be described by means of the Rayleigh equation. In this case, isotopic effects will always be expressed as a constant, hence will not be able to describe isotopic effects in reactions where fractionation and enrichment are variable or inverse during the course of a reaction. Most chemical reactions do not follow first-order kinetics; neither biochemical reactions can normally be described with first-order kinetics. To properly describe isotopic effects in chemical or biochemical reactions, different approaches must be employed such as the use of Michaelis-Menten reaction order (for chemical reactions) or coupled Michaelis-Menten and Monod reaction orders (for biochemical reactions). However, conversely to Michaelis-Menten kinetics, GEBIK and GEBIF equations are solved under the hypothesis of non steady state. This characteristic allows GEBIK and GEBIF to capture transient isotopic effects.
Mathematical description of transient kinetic isotope effects
The GEBIK and GEBIF equations are introduced here below.Notation
The GEBIK and GEBIF equations describe the dynamics of the following state variables substrate concentration
substrate concentration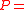 product concentration
product concentration enzyme concentration
enzyme concentration complex concentration
complex concentration biomass concentration
biomass concentrationBoth S and P contain at least one isotopic expression of a tracer atom. For instance, if the carbon element is used as a tracer, both S and P contain at least one C atom, which may appear as
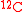 and
and 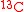 . The isotopic expression within a molecule is
. The isotopic expression within a molecule is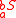
where
 is the number of tracer atoms within S, while
is the number of tracer atoms within S, while  is the number of isotopic substitutions in the same molecule. The condition
is the number of isotopic substitutions in the same molecule. The condition  must be satisfied. For example, the N
must be satisfied. For example, the N product in which 1 isotopic substitution occurs (e.g.,
product in which 1 isotopic substitution occurs (e.g., 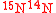 ) will be described by
) will be described by 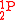 .
.Substrates and products appear in a chemical reaction with specific stoichiometric coefficients. When chemical reactions comprise combinations of reactants and products with various isotopic expressions, the stoichiometric coefficients are functions of the isotope substitution number. If
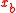 and
and  are the stoichiometric coefficient for
are the stoichiometric coefficient for 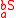 substrate and
substrate and 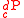 product, a reaction takes the form
product, a reaction takes the form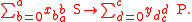
For example, in the reaction

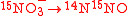 , the notation is
, the notation is 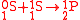 with
with 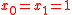 for both isotopologue reactants of the same substrate with substitution number
for both isotopologue reactants of the same substrate with substitution number  and
and  , and with
, and with 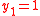 for
for 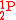 and
and 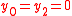 because the reaction does not comprise production of
because the reaction does not comprise production of 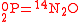 and
and 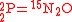 .
.For isotopomers, the substitution location is taken into account as
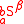 and
and 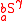 , where
, where 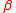 and
and  indicate a different expressions of the same isotopologue
indicate a different expressions of the same isotopologue 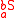 . Isotopomers only exist when
. Isotopomers only exist when  and
and  . The substitution location has to be specifically defined depending on the number of tracer atoms
. The substitution location has to be specifically defined depending on the number of tracer atoms  , number of substitutions
, number of substitutions 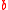 , and molecule structure. For multiatomic molecules that are symmetric with respect to tracer position, there is no need to specify the substitution position when
, and molecule structure. For multiatomic molecules that are symmetric with respect to tracer position, there is no need to specify the substitution position when  . For example, one substitution of deuterium D =
. For example, one substitution of deuterium D =  in the symmetric methane molecule CDH
in the symmetric methane molecule CDH does not require the use of the right superscript. In the case that
does not require the use of the right superscript. In the case that  , the substitution location has to be specified, while for CHD
, the substitution location has to be specified, while for CHD and CD
and CD it is not required. For example, two D substitutions in CD
it is not required. For example, two D substitutions in CD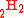 can occur in adjacent or non-adjacent locations. Using this notation, the reaction
can occur in adjacent or non-adjacent locations. Using this notation, the reaction  can be written as
can be written as
where
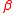 in
in 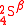 defines only one of the two methane forms (either with adjacent or non-adjacent D atoms). The location of D in the two isotopologue water molecules produced on the right-hand side of the reaction has not been indicated because D is present in only one water molecule at saturation, and because the water molecule is symmetric. For asymmetric and multiatomic molecules with
defines only one of the two methane forms (either with adjacent or non-adjacent D atoms). The location of D in the two isotopologue water molecules produced on the right-hand side of the reaction has not been indicated because D is present in only one water molecule at saturation, and because the water molecule is symmetric. For asymmetric and multiatomic molecules with  and
and  , definition of the substitution location is always required. For instance, the isotopomers of the (asymmetric) nitrous oxide molecule
, definition of the substitution location is always required. For instance, the isotopomers of the (asymmetric) nitrous oxide molecule 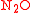 are
are 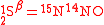 and
and 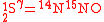 .
.Reactions of asymmetric isotopomers can be written using the partitioning coefficient
 as
as
where
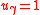 . For example, using N isotope tracers, the isotopomer reactions
. For example, using N isotope tracers, the isotopomer reactions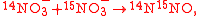
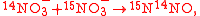
can be written as one reaction in which each isotopomer product is multiplied by its partition coefficient as
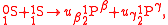
with
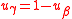 . More generally, the tracer element does not necessarily occur in only one substrate and one product. If
. More generally, the tracer element does not necessarily occur in only one substrate and one product. If 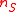 substrates react releasing
substrates react releasing 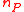 products, each having an isotopic expression of the tracer element, then the generilized reaction notation is
products, each having an isotopic expression of the tracer element, then the generilized reaction notation is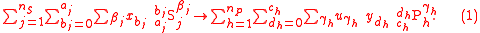
For instance, consider the
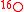 and
and 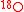 tracers in the reaction
tracers in the reaction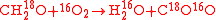
In this case the reaction can be written as

with two substrates and two products without indication of the substitution location because all molecules are symmetric.
Biochemical kinetic reactions of type (1) are often catalytic reactions in which one or more substrates,
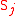 , bind to an enzyme, E, to form a reversible activated complex, C, which releases one or more
, bind to an enzyme, E, to form a reversible activated complex, C, which releases one or moreproducts,
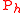 , and free, unchanged enzyme. These reactions belong to the type of reactions that can be described by Michaelis-Menten
, and free, unchanged enzyme. These reactions belong to the type of reactions that can be described by Michaelis-MentenMichaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
kinetics. Using this approach for substrate and product isotopologue and isotopomer expressions, and under the prescribed stoichiometric relationships among them, leads to the general reactions of the Michaelis-Menten type

with the index
 , where
, where  depends on the number of possible atomic combinations among all isotopologues and isotopomers. Here,
depends on the number of possible atomic combinations among all isotopologues and isotopomers. Here, 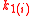 ,
,  , and
, and  are the rate constants indexed for each of the m reactions.
are the rate constants indexed for each of the m reactions.Example
The reactions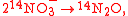
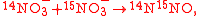
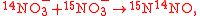
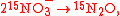
can be written as
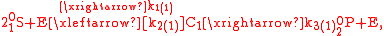


Isotope mass balance
The following isotope mass balances must hold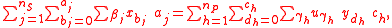

General Equations for Biochemical Isotope Kinetics (GEBIK)
To solve for the concentration of all components appearing in any general biochemical reaction as in (2), the Michaelis-Menten kinetics for an enzymatic reaction are coupled with the Monod kinetics for biomass dynamics. The most general case is to assume that the enzyme concentration is proportional to the biomass concentration and that the reaction is not in quasi-steady state. These hypotheses lead to the following system of equations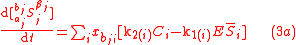
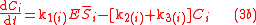
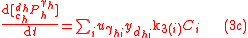
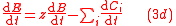
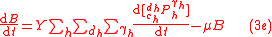 .
.with
 , and where
, and where 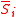 is the concentration of the most limiting substrate in each reaction i, z is the enzyme yield coefficient, Y is the yield coefficient expressing the biomass gain per unit of released product and
is the concentration of the most limiting substrate in each reaction i, z is the enzyme yield coefficient, Y is the yield coefficient expressing the biomass gain per unit of released product and  is the biomass mortality rate.
is the biomass mortality rate.General Equations for Biochemical Isoptope Fractionation (GEBIF)
The isotopic composition of the components in a biochemical system can be defined in different ways depending on the definition of isotopic ratio. Three definitions are described here:Isotopic ratio - definition 1
Isotopic ratio relative to each component in the system, each with its isotopic expression, with respect to the concentration of its most abundantisotopologue
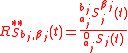
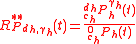
Isotopic ratio - definition 2
Isotopic ratio relative to the mass of the tracer element in each component;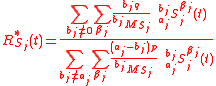
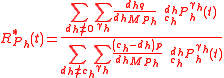
where ,
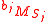 and
and 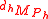 are the molecular weight of each isotopic expression of the substrate and product.
are the molecular weight of each isotopic expression of the substrate and product.Isotopic ratio - definition 3
Isotopic ratio relative to the mass of the tracer element in the accumulatedsubstrates and products
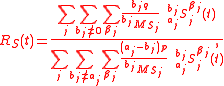
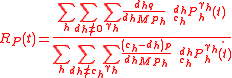
Isotopic composition
Regardless of the definition of the isotopic ratio, the isotopic composition of substrate and product are expressed as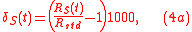
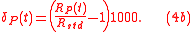
where
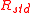 is a standard isotopic ration. Here, definition 3 of isotopic ratio has been used, however, any of the three definitions of isotopic ratio can equally be used.
is a standard isotopic ration. Here, definition 3 of isotopic ratio has been used, however, any of the three definitions of isotopic ratio can equally be used.Fractionation factor
The isotopic ratio of the product can be used to define the instantaneousisotopic ratio

and the time-dependent fractionation factor
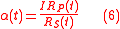
Isotopic enrichment
The time-dependent isotopic enrichment is simply defined as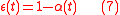
Simplified forms of GEBIK and GEBIF
Under specific assumptions, the GEBIK and GEBIF equations become equivalent to the equation for steady-state kinetic isotope fractionation in both chemical and biochemical reactions. Here two mathematical treatments are proposed: (i) under biomass-free and enzyme-invariant (BFEI) hypothesis and (ii) under quasi-steady-state (QSS) hypothesis.BFEI hypothesis
In instances where the biomass and enzyme concentrations are not appreciably changing in time, we can assume that biomass dynamics is negligible and that the total enzyme concentration is constant, and the GEBIK equations become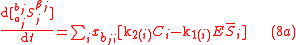
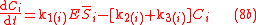
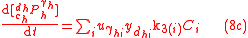
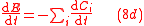
Eqs. (4) for isotopic compositions, Eq. (6) for the fractionation factor and Eq. (7) for the enrichment factor equally applies to the GEBIK equations under the BFEI hypothesis.
QSS hypothesis
If the quasi-steady-state hypothesis is assumed in addition to BFEI hypothesis, then the complex concentration can be assumed to be in a stationary (steady) state according to the Briggs-Haldane hypothesis, and the GEBIK equations become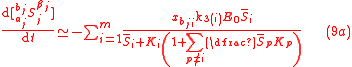
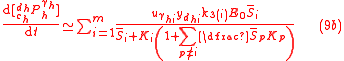
which are written in a form similar to the classical Micaelis-Menten equations for any substrate and product. Here, the equations also show that the various isotopologue and isotopomer substrates appear as competing species. Eqs. (4) for isotopic compositions, Eq. (6) for the fractionation factor and Eq. (7) for the enrichment factor equally applies to the GEBIK equations under the BFEI and QSS hypothesis.
Example of application of GEBIK and GEBIF
An example is shown where GEBIK and GEBIF equations are used to describe the isotopic reactions of N O consumption into N
O consumption into N according to the simultaneous set of reactions
according to the simultaneous set of reactions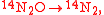
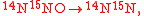
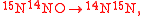
These can be rewritten using the notation introduced before as.
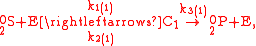
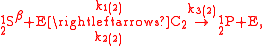
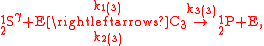
The substrate
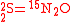 has not been included due to its scarcity. In addition, we have not specified the isotopic substitution in the N
has not been included due to its scarcity. In addition, we have not specified the isotopic substitution in the N product of the second and third reactions because N
product of the second and third reactions because N is symmetric. Assuming that the second and third reactions have identical reaction rates
is symmetric. Assuming that the second and third reactions have identical reaction rates  ,
,  , and
, and  , the full GEBIK and GEBIF equations are
, the full GEBIK and GEBIF equations are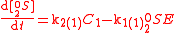
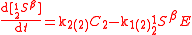
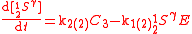
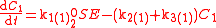
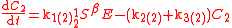
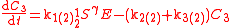
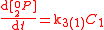

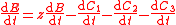
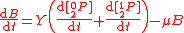
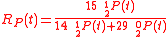

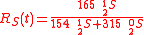
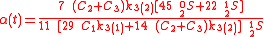
Example of application of GEBIK and GEBIF under BFEI and QSS hypotheses
The same reaction can be described with the GEBIK and GEBIF equations under the BFEI and QSS approximations as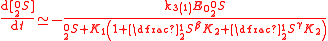

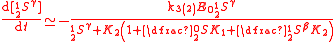

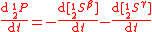
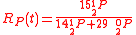
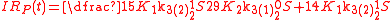
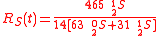
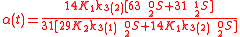
where K
 has been substituted with K
has been substituted with K because the rate
because the rateconstants in the third reaction have been assumed to equal those
of the second reaction.

