
Three-center four-electron bond
Encyclopedia
The 3-center 4-electron bond is a model used to explain bonding in hypervalent molecule
s such as phosphorus pentafluoride
, sulfur hexafluoride
, the xenon fluorides, and the bifluoride ion. It is also known as the Pimentel-Rundle three-center model after the work published by George C. Pimentel
in 1951, which built on concepts developed earlier by Robert E. Rundle for electron-deficient bonding.
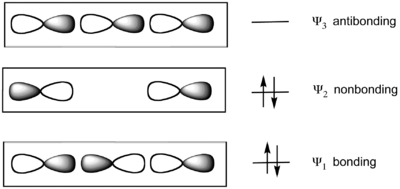
The model considers bonding of three colinear atoms. For example in XeF2, the linear F−Xe−F subunit is described by a set of three molecular orbitals (MOs) derived from colinear p-orbitals on each atom. The Xe−F bonds result from the combination of a filled p orbital in the central atom (Xe) with two half-filled p orbitals on the axial atoms (F), resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding
orbital. The two lower energy MO's are doubly occupied. The HOMO
is localized on the two terminal atoms. This localization of charge is accommodated by the fact that the terminal ligands are highly electronegative in hypervalent molecules. The molecules PF5 and SF4 are described, according to this model, as having one 3-center 4-electron bond as well as three and two other more conventionally described bonds, respectively. In SF6 and in the xenon fluorides, all bonds are described with the 3-center-4-electron model.
 The bonding in XeF2 can also be shown qualitatively using resonant
The bonding in XeF2 can also be shown qualitatively using resonant
Lewis structure
s as shown below:

In this representation, the octet rule
is not broken, the bond order
s are 1/2, and there is increased electron density in the fluorine atoms. These results are consistent with the molecular orbital picture discussed above.
Older models for explaining hypervalency invoked d orbitals. As of 2010, these models still appear in some beginning-level college texts; however, quantum chemical
calculations suggest that d-orbital participation is negligible due to the large energy difference between the relevant p (filled) and d (empty) orbitals. Furthermore, a distinction should be made between "d orbitals" in the valence bond sense and "d functions" that are included in the QM calculation as polarization functions. The 3-center-4-electron bonding model has the advantage of dispensing with the need for d orbitals, which has led to its acceptance.
Three-center four-electron interactions can also be considered in the transition state of SN2 reaction
s and in some (resonant) hydrogen bonding:

Hypervalent molecule
A hypervalent molecule is a molecule that contains one or more main group elements formally bearing more than eight electrons in their valence shells...
s such as phosphorus pentafluoride
Phosphorus pentafluoride
Phosphorus pentafluoride, PF5, is a phosphorus halide. It's a colourless gas at room temperature and pressure.-Structure:Single-crystal X-ray studies indicate PF5 molecule has two distinct P−F bonds : P−Fax = 158.0 pm and P−Feq = 152.2 pm...
, sulfur hexafluoride
Sulfur hexafluoride
Sulfur hexafluoride is an inorganic, colorless, odorless, and non-flammable greenhouse gas. has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule. Typical for a nonpolar gas, it is poorly soluble in water but soluble in...
, the xenon fluorides, and the bifluoride ion. It is also known as the Pimentel-Rundle three-center model after the work published by George C. Pimentel
George C. Pimentel
George Claude Pimentel was the inventor of the chemical laser. He also developed the modern technique of matrix isolation in low-temperature chemistry. In theoretical chemistry, he proposed the three-centre four-electron bond which is now accepted as the best simple model of hypervalent...
in 1951, which built on concepts developed earlier by Robert E. Rundle for electron-deficient bonding.
The model considers bonding of three colinear atoms. For example in XeF2, the linear F−Xe−F subunit is described by a set of three molecular orbitals (MOs) derived from colinear p-orbitals on each atom. The Xe−F bonds result from the combination of a filled p orbital in the central atom (Xe) with two half-filled p orbitals on the axial atoms (F), resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
orbital. The two lower energy MO's are doubly occupied. The HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
is localized on the two terminal atoms. This localization of charge is accommodated by the fact that the terminal ligands are highly electronegative in hypervalent molecules. The molecules PF5 and SF4 are described, according to this model, as having one 3-center 4-electron bond as well as three and two other more conventionally described bonds, respectively. In SF6 and in the xenon fluorides, all bonds are described with the 3-center-4-electron model.

Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
Lewis structure
Lewis structure
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds...
s as shown below:

In this representation, the octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
is not broken, the bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
s are 1/2, and there is increased electron density in the fluorine atoms. These results are consistent with the molecular orbital picture discussed above.
Older models for explaining hypervalency invoked d orbitals. As of 2010, these models still appear in some beginning-level college texts; however, quantum chemical
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
calculations suggest that d-orbital participation is negligible due to the large energy difference between the relevant p (filled) and d (empty) orbitals. Furthermore, a distinction should be made between "d orbitals" in the valence bond sense and "d functions" that are included in the QM calculation as polarization functions. The 3-center-4-electron bonding model has the advantage of dispensing with the need for d orbitals, which has led to its acceptance.
Three-center four-electron interactions can also be considered in the transition state of SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
s and in some (resonant) hydrogen bonding:


