
Thomson (unit)
Encyclopedia
The thomson is a unit that has appeared infrequently in scientific literature relating to the field of mass spectrometry
as a unit of mass-to-charge ratio
. The unit was proposed by Cooks and Rockwood naming it in honour of J. J. Thomson
who measured the mass-to-charge ratio
of electrons and ions.
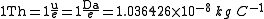
where u represents the unified atomic mass unit, Da represents the unit dalton, and e represents the elementary charge
which is the electric charge
unit
in the atomic unit system.
For example, for the ion C7H72+ has an exact mass of 91.0 Da. Its charge number is +2, and hence its charge is 2e. The ion will be observed at 45.5 Th in a mass spectrum.
The thomson allows for negative values for negatively charged ions. For example, the benzoate anion would be observed at m/z 121, but at −121 Th since the charge is −e.
—the inventor of the Orbitrap
—in a scientific poster, papers,
and (notably) one book. The journal Rapid Communications in Mass Spectrometry
(in which the original article appeared) states that "the Thomson (Th) may be used for such purposes as a unit of mass-to-charge ratio although it is not currently approved by IUPAP or IUPAC." Even so, the term has been called "controversial" by RCM's former Editor-in Chief (in a review the Hoffman text cited above). The book, Mass Spectrometry Desk Reference, argues against the use of the thomson. However, the editor-in-chief of the Journal of the Mass Spectrometry Society of Japan has written an editorial in support of the thomson unit.
The thomson is not an SI unit, nor has it been defined by IUPAC.
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
as a unit of mass-to-charge ratio
Mass-to-charge ratio
The mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
. The unit was proposed by Cooks and Rockwood naming it in honour of J. J. Thomson
J. J. Thomson
Sir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
who measured the mass-to-charge ratio
Mass-to-charge ratio
The mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
of electrons and ions.
Definition
The thomson is defined as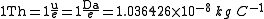
where u represents the unified atomic mass unit, Da represents the unit dalton, and e represents the elementary charge
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
which is the electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
unit
Units of measurement
A unit of measurement is a definite magnitude of a physical quantity, defined and adopted by convention and/or by law, that is used as a standard for measurement of the same physical quantity. Any other value of the physical quantity can be expressed as a simple multiple of the unit of...
in the atomic unit system.
For example, for the ion C7H72+ has an exact mass of 91.0 Da. Its charge number is +2, and hence its charge is 2e. The ion will be observed at 45.5 Th in a mass spectrum.
The thomson allows for negative values for negatively charged ions. For example, the benzoate anion would be observed at m/z 121, but at −121 Th since the charge is −e.
Use
The thomson has been used by some mass spectrometrists, for example Alexander MakarovAlexander Alexeyevich Makarov
Alexander Alexeyevich Makarov is a Russian physicist who led the team that developed the Orbitrap, a type of mass spectrometer, and received the 2008 American Society for Mass Spectrometry Distinguished Contribution in Mass Spectrometry Award for this development.- Early life and education :* 1989...
—the inventor of the Orbitrap
Orbitrap
An orbitrap is a type of mass spectrometer invented by Alexander Makarov. It consists of an outer barrel-like electrode and a coaxial inner spindle-like electrode that form an electrostatic field with quadro-logarithmic potential distribution....
—in a scientific poster, papers,
and (notably) one book. The journal Rapid Communications in Mass Spectrometry
Rapid Communications in Mass Spectrometry
Rapid Communications in Mass Spectrometry , is a peer-reviewed scientific journal, published since 1987 by John Wiley & Sons...
(in which the original article appeared) states that "the Thomson (Th) may be used for such purposes as a unit of mass-to-charge ratio although it is not currently approved by IUPAP or IUPAC." Even so, the term has been called "controversial" by RCM's former Editor-in Chief (in a review the Hoffman text cited above). The book, Mass Spectrometry Desk Reference, argues against the use of the thomson. However, the editor-in-chief of the Journal of the Mass Spectrometry Society of Japan has written an editorial in support of the thomson unit.
The thomson is not an SI unit, nor has it been defined by IUPAC.

