Temperature jump is a technique used in the study of
chemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
. It usually involves the discharging of a capacitor (in the kV range) through a small volume (
absorption spectroscopyAbsorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
or fluorescence spectroscopyFluorescence spectroscopy aka fluorometry or spectrofluorometry, is a type of electromagnetic spectroscopy which analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit...
. Due to the small volumes involved the temperature of the solution returns to that of its surroundings in minutes.
The fractional extent of the reaction (i.e. the percentage change in concentration of a measurable species) depends on the molar enthalpy change (ΔH°) between the reactants and products and the equilibrium position. If K is the equilibrium constant and dT is the change in temperature then the enthalpy change is given by the Van't Hoff equation:
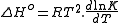
where R is the universal gas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the absolute temperatureThermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
. When a single step in a reaction is perturbed in a temperature jump experiment, the reaction follows a single exponential decay function with the reciprocal time constantIn physics and engineering, the time constant, usually denoted by the Greek letter \tau , is the risetime characterizing the response to a time-varying input of a first-order, linear time-invariant system.Concretely, a first-order LTI system is a system that can be modeled by a single first order...
(1/τ) equal to the sum of the forward and reverse intrinsic rate constants. In more complex reaction networks, when multiple reaction steps are perturbed, then the reciprocal time constants are given by the eigenvalues of the characteristic rate equations. The ability to observe intermediate steps in a reaction pathway is one of the attractive features of this technology.
The source of this article is
wikipedia, the free encyclopedia. The text of this article is licensed under the
GFDL.