
Standard enthalpy change of reaction
Encyclopedia
The standard enthalpy of reaction (denoted ΔrH⊖) is the enthalpy
change that occurs in a system when one mole of matter is transformed by a chemical reaction
under standard conditions.
For a generic chemical reaction
the standard enthalpy of reaction ΔrH⊖ is related to the standard enthalpy of formation ΔfHo of the reactants and products by the following equation:
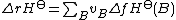
In this equation, vB is the stoichiometric coefficient of entity B.
A similar enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. The enthalpy change of any reaction under any conditions can be computed, given the standard enthalpy of formation of the reactants and product
s.
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change that occurs in a system when one mole of matter is transformed by a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
under standard conditions.
For a generic chemical reaction
- −vA A + −vB B + ... → vP P + vQ Q ...
the standard enthalpy of reaction ΔrH⊖ is related to the standard enthalpy of formation ΔfHo of the reactants and products by the following equation:
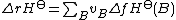
In this equation, vB is the stoichiometric coefficient of entity B.
A similar enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. The enthalpy change of any reaction under any conditions can be computed, given the standard enthalpy of formation of the reactants and product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
s.

