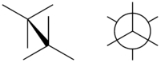
Staggered
Encyclopedia
In organic chemistry, a staggered conformation is a chemical conformation of an ethane
-like moiety
abcX-Ydef in which the substituents a,b,and c are at the maximum distance from d,e,and f. This requires the torsion angles to be 60°.
Such a conformation exists in any open chain single chemical bond
connecting two sp3 hybridised
atoms, and is normally a conformational energy minimum.For some molecule
s such as those of n-butane
, there can be special versions of staggered conformations called gauche and anti; see first Newman projection
diagram in Conformational isomerism
.
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
-like moiety
Moiety
Moiety may refer to:* Moiety , a part or functional group of a molecule* Moiety , either of two groups into which a society is divided* An Australian Aboriginal kinship group* Native Hawaiian realm ruled by a Mo'i or Ali'i...
abcX-Ydef in which the substituents a,b,and c are at the maximum distance from d,e,and f. This requires the torsion angles to be 60°.
Such a conformation exists in any open chain single chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
connecting two sp3 hybridised
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
atoms, and is normally a conformational energy minimum.For some molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s such as those of n-butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
, there can be special versions of staggered conformations called gauche and anti; see first Newman projection
Newman projection
A Newman projection, useful in alkane stereochemistry, visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle . The front carbon atom is called proximal, while the back atom is called distal...
diagram in Conformational isomerism
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
.

