
Solenopsin
Encyclopedia
Solenopsin is an alkaloid
which inhibits angiogenesis
via the phosphoinositol-3 kinase (PI3-K) signaling pathway, in addition to contributing to the toxic effect of fire ant
venom. Solenopsin has also been shown to have cytotoxic, hemolytic, necrotic, insecticidal, antibacterial, antifungal, and anti-HIV
properties. Originally synthesized in 1998, several groups have designed novel and creative methods of synthesizing enantiopure solenopsin and other alkaloidal components of ant venom. Though the use of ant venom in a remedial sense has often been marginalized to homeopathic therapies, basic science research is just beginning to uncover the therapeutic value of these alkaloids in human pathologies.
Figure 1
Summarized schematic of the total solenopsin synthesis
In addition to these anti-angiogenic effects, solenopsin potently inhibits the neuronal nitric oxide synthase
(nNOS) in a manner that appears to be non-competitive with L-arginine. "The nNOS inhibition by solenopsin compares favorably with the inhibitory potency of widely used nNOS inhibitors."
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
which inhibits angiogenesis
Angiogenesis
Angiogenesis is the physiological process involving the growth of new blood vessels from pre-existing vessels. Though there has been some debate over terminology, vasculogenesis is the term used for spontaneous blood-vessel formation, and intussusception is the term for the formation of new blood...
via the phosphoinositol-3 kinase (PI3-K) signaling pathway, in addition to contributing to the toxic effect of fire ant
Fire ant
Fire ants are a variety of stinging ants with over 285 species worldwide. They have several common names, including ginger ants, tropical fire ants and red ants.- Appearance :...
venom. Solenopsin has also been shown to have cytotoxic, hemolytic, necrotic, insecticidal, antibacterial, antifungal, and anti-HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
properties. Originally synthesized in 1998, several groups have designed novel and creative methods of synthesizing enantiopure solenopsin and other alkaloidal components of ant venom. Though the use of ant venom in a remedial sense has often been marginalized to homeopathic therapies, basic science research is just beginning to uncover the therapeutic value of these alkaloids in human pathologies.
Synthesis
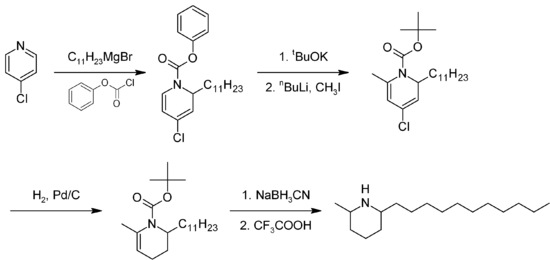
The total synthesis of solenopsin has been described by several methods. The method proposed by Bowen et al.(Figure 1) starts with alkylation of 4-chloropyridine hydrochloride with a 1-bromoundecane pre-formed Grignard reagent, followed by amidation of the pyridinal nitrogen with phenyl chloroformate, to form 4-Chloro-1-(phenoxycarbonyl)-2-n-undecyl-1,2-dihydropyridine. Phenol is the displaced by t-butoxide, and the pyridine is then methylated at the 6 position. The pyridine ring is then reduced to a 1,2,3,4-tetrahydropyridine via catalytic hydrogenation over palladium. Boc is finally removed to yield (+)-Solenopsin A as trans-2-methyl-6-n-undecylpiperidine. The hydrochloride salt is then formed with addition of HCl and concentration of the solution yields pure Solenopsin A HCl as a white solid. A number of analogs have been synthesized using modifications of this procedure, but Solenopsin A has been shown to have the most potent anti-angiogenic effects.Figure 1

Summarized schematic of the total solenopsin synthesis
Biological activity
In vitro studies in embryonic zebrafish in the lab of Jack L. Arbiser at Emory University first revealed solenopsin's anti-angiogenic effects. Further studies have shown that solenopsin inhibits the survival protein Akt in an ATP-competitive manner, without affecting upstream activators PI3-K dependent protein kinase 1 (PDK1) or PI3-K itself. While the direct protein target of solenopsin has not yet been identified, current research suggests a target upstream of PI3-K.In addition to these anti-angiogenic effects, solenopsin potently inhibits the neuronal nitric oxide synthase
Nitric oxide synthase
Nitric oxide synthases are a family of enzymes that catalyze the production of nitric oxide from L-arginine. NO is an important cellular signaling molecule, having a vital role in many biological processes...
(nNOS) in a manner that appears to be non-competitive with L-arginine. "The nNOS inhibition by solenopsin compares favorably with the inhibitory potency of widely used nNOS inhibitors."

