
Sodium tungsten bronze
Encyclopedia
Sodium tungsten bronze is a form of insertion compound
with the formula NaxWO3, where x is equal to or less than 1. Named due to its metallic lustre, its electrical properties range from semiconducting to metallic depending on the concentration of sodium ions present; it can also exhibit superconductivity
.
, sodium tungsten bronze was the first alkali metal bronze to be discovered.
Because of the relative stability of the tungsten(V)
cation that is formed, it was not until the 1960s that molybdenum
bronzes were discovered.
The electrical resistivity of the bronze depends on the proportion of sodium in the compound, with specific resistances of 1.66 mΩ being measured for some samples. It has been suggested that electrons, released when the sodium atoms are ionised, are conducted readily through the tungsten t2g and oxygen π orbitals. This can be observed in the XPS
and UPS
spectra: the peak representing the tungsten 5d band becomes more intense as x rises.
For values of x below 0.3, the bronze is semiconducting rather than metallic. When cooled sufficiently, sodium tungsten bronze becomes a superconductor, with the critical temperature (Tc) for Na0.23WO3 being approximately 2.2 kelvin
. The first record of superconductivity in a tungsten bronze was in 1964, with a Tc of 0.57 K.
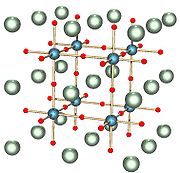 When x=1, sodium tungsten bronze adopts a cubic phase: the perovskite crystal structure. In this form, the structure consists of corner-sharing WO6 octahedra with sodium ions in the interstitial gaps. For x values between 0.9 and 0.3, the structure remains similar but with an increasing deficiency of sodium ions and a smaller lattice parameter.
When x=1, sodium tungsten bronze adopts a cubic phase: the perovskite crystal structure. In this form, the structure consists of corner-sharing WO6 octahedra with sodium ions in the interstitial gaps. For x values between 0.9 and 0.3, the structure remains similar but with an increasing deficiency of sodium ions and a smaller lattice parameter.
A number of other structure types can also be adopted, with varying electrical properties: cubic, tetragonal I and hexagonal phases are metallic, whereas orthorhombic and tetragonal II structures are semiconducting.
and tungsten trioxide
with hydrogen gas at red heat. A more modern approach reduces a melt of the reactants with electricity rather than with hydrogen. Microwave synthesis is also possible, using tungsten powder as the reducing agent.
Intercalation (chemistry)
In chemistry, intercalation is the reversible inclusion of a molecule between two other molecules . Examples include DNA intercalation and graphite intercalation compounds.- DNA intercalation :...
with the formula NaxWO3, where x is equal to or less than 1. Named due to its metallic lustre, its electrical properties range from semiconducting to metallic depending on the concentration of sodium ions present; it can also exhibit superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
.
History
Prepared in 1823 by the chemist Friedrich WöhlerFriedrich Wöhler
Friedrich Wöhler was a German chemist, best known for his synthesis of urea, but also the first to isolate several chemical elements.-Biography:He was born in Eschersheim, which belonged to aau...
, sodium tungsten bronze was the first alkali metal bronze to be discovered.
Because of the relative stability of the tungsten(V)
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
cation that is formed, it was not until the 1960s that molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
bronzes were discovered.
Properties
Sodium tungsten bronze, like other tungsten bronzes, is resistant to chemical reaction under both acidic and basic conditions. Colour is dependent upon the proportion of sodium in the compound, ranging from golden at x≈0.9, through red, orange and deep purple, to blue-black when x≈0.3.The electrical resistivity of the bronze depends on the proportion of sodium in the compound, with specific resistances of 1.66 mΩ being measured for some samples. It has been suggested that electrons, released when the sodium atoms are ionised, are conducted readily through the tungsten t2g and oxygen π orbitals. This can be observed in the XPS
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
and UPS
Ultraviolet photoelectron spectroscopy
Ultraviolet photoelectron spectroscopy refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules which have absorbed ultraviolet photons, in order to determine molecular energy levels in the valence region.-Basic Theory:...
spectra: the peak representing the tungsten 5d band becomes more intense as x rises.
For values of x below 0.3, the bronze is semiconducting rather than metallic. When cooled sufficiently, sodium tungsten bronze becomes a superconductor, with the critical temperature (Tc) for Na0.23WO3 being approximately 2.2 kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. The first record of superconductivity in a tungsten bronze was in 1964, with a Tc of 0.57 K.
Structure

A number of other structure types can also be adopted, with varying electrical properties: cubic, tetragonal I and hexagonal phases are metallic, whereas orthorhombic and tetragonal II structures are semiconducting.
Synthesis
Wöhler's 1823 synthesis involved reducing sodium tungstateSodium tungstate
Sodium tungstate, Na2WO4, a tungstate of sodium, is useful as a source of tungsten. It is prepared from tungsten ores used to manufacture tungsten by reducing it....
and tungsten trioxide
Tungsten trioxide
Tungsten oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. Tungsten ores are treated with alkalis to produce WO3...
with hydrogen gas at red heat. A more modern approach reduces a melt of the reactants with electricity rather than with hydrogen. Microwave synthesis is also possible, using tungsten powder as the reducing agent.

