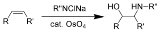
Sharpless oxyamination
Encyclopedia
The Sharpless oxyamination (often known as Sharpless aminohydroxylation) is the chemical reaction
of alkene
s with alkyl imido osmium
compounds to form vicinal amino-alcohols. A comprehensive review of this reaction was authored by McLeod et al. in 2002.
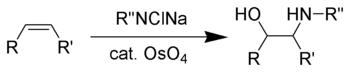 Vicinal amino-alcohols are important products in organic synthesis
Vicinal amino-alcohols are important products in organic synthesis
and recurring pharmacophore
s in drug discovery
.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s with alkyl imido osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
compounds to form vicinal amino-alcohols. A comprehensive review of this reaction was authored by McLeod et al. in 2002.

Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
and recurring pharmacophore
Pharmacophore
thumb|right|300px|An example of a pharmacophore model.A pharmacophore is an abstract description of molecular features which are necessary for molecular recognition of a ligand by a biological macromolecule....
s in drug discovery
Drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
.

